Research Paper - Journal of Fisheries Research (2021) Volume 5, Issue 4
Evaluation of growth, survival and carcass composition of fish and prawn in the substrate based polyculture farming.
Aditi Rajan Patkar1*, Manjappa N2, Harsha Nayak2
1Department of Aquaculture, Karnataka Veterinary Animal and Fisheries Sciences University, Bidar, India
2Fisheries Research and Information Centre (Inland), Karnataka Veterinary Animal and Fisheries Sciences University, Bidar, India
- Corresponding Author:
- Aditi Rajan Patkar Department of Aquaculture, Karnataka Veterinary Animal and Fisheries Sciences University Bidar India E-mail: adipatkar@gmail.com
Accepted date: April 7, 2020
Citation: Farvardin M, Johari MK, Nami M, et al. Annular choroidal detachment one year after argus-II retinal prosthes is implantation. Ophthalmol Case Rep. 2020;4(1):23-25.
Abstract
A series of substrate based aquaculture systems have been developed to reduce the cost of finfish and shellfish farming. Periphyton acts as important food component for finfish and shellfishes. The present study is aimed to assess the growth and survival of fish (Labeo fimbriatus, Barbodes carnaticus) and shell fish (Macrobrachium rosenbergii) in a substrate based system for a period of 6 months. Sugarcane bagasse is used as a substrate for the growth of periphytons. The experiment was carried out in three treatments, T1 (only substrate), T2 (substrate + feed) and T0 (only feed/ control) with triplicates. The results of the study showed maximum average weight in T2 ( L. fimbriatus 141.17Keywords
Fish and shellfish, Polyculture, Water quality, Sugarcane bagasse, Periphyton
Introduction
Over the years, the semi-intensive systems of fish farming are highly subjected to use of feed and fertilizers. However, these leads to increase in the cost of production and most of the time given food is not consumed by animal and finally goes to environment as fish waste when it is discharged into the water bodies causes problem of eutrophication [1,2]. Also, high feed costs are unbearable for small and marginal fish farmers. Therefore, from the economic and environmental points of view, there is a need to make semi-intensive aquaculture systems more nutrient-efficient.
In any aquatic system, autotrophic and heterotrophic are two important feeding pathways for fish, as they play major role in the nutrient cycling as well as carbon flow in water column. In most of the culture systems, two basic food sources present for all organisms viz., primary productivity from algae, where secondary trophic feeders such as zooplankton, benthos including fish utilizes organic matter produced by algae. Secondly, the organic matter added as feed or manures, to enhance productivity by direct or indirect consumption by fish. In both cases, heterotrophic microorganisms (bacteria, fungi, protozoa) are essentially important where they decompose organic matter and releases nutrients which either utilized by algae or taken by cultured organism [3]. One can reduce the cost of artificial feed via enhancing autotrophic and heterotrophic production in culture pond by raising fertilization rate. However, autotrophs are light dependent and promotion of heterotrophs in the pond can be carried out during night time by using different substrate via periphyton technology [4].
Periphyton is considered as an important food component for finfishes and shellfishes. It is being used traditionally as rich aquatic feed for fishes throughout the countries like Cambodia, West Africa, Srilanka, India and Bangladesh. The principle of periphyton-based aquaculture is to provide substrates on which bacteria, protozoa, fungi, phytoplankton, zooplankton, benthic organisms, and a range of other invertebrates colonize and which acts as source of feed for fishes. This periphyton, traps dissolved and suspended nutrients from the water matrix and in turn, increases natural feed to cultured organisms. Periphyton is effectively utilized by many fish species which thrive low in the food chain [5, 6]. Along with food production, periphyton acts as water purifier by means of nutrient cycling and retention of nitrogenous compounds [7]. Besides, substrates are shelters for avoiding agonistic behavior in prawns, which ultimately increases yield [8].
Many researchers have demonstrated the use of different substrates such as bamboo for Tor khudree, Labeo fimbriatus, Oreochromis niloticus and Macrobrachium rosenbergii, plastic sheet and ceramic tile for brackishwater shrimp, rice straw mats for Nile tilapia and sugarcane bagasse for Labeo rohita and Etroplus suratensis, to improve water quality, enhance growth and increase production [9-14]. Sayed stated that periphyton can partially or totally replace or complement supplemental feed in phytophagous tilapia ponds, without reducing fish yield but with considerable reduction in production cost. Thus, this aquaculture technique can be an excellent alternative to reduce production cost and allow an economically viable fish production, particularly in rural, resource limited regions in developing countries [15].
Furthermore, there is a great scope for enhancement of heterotrophic food production by using easily available agricultural wastes like sugarcane bagasse, paddy straw along with fertilizers/manures, these serves two important purposes viz., it can be converted to protein rich microbial biomass and at the same time reduces the problem of waste disposal[16]. Gangadhar and Keshavanath evaluated bagasse was good periphyton substrates in terms of periphyton ash free dry matter, chlorophyll-a and nutrient composition. The earlier studies were based on the viability of periphyton-based aquaculture technology and there is very less work on the impact of these substrates on composition of fish body composition. The present study was aimed to study the influence sugarcane bagasse on growth, survival and carcass composition of Labeo fimbriatus, Barbodes carnaticus and Macrobrachium rosenbergii in polyculture system.
Materials and Methods
Experimental design
The experiment was conducted for a period of 180 days at the Fisheries Research Information Centre (Inland), Hesaraghatta (13°8’18.8088”N and 77°28’40.4040” E), Bengaluru, and Karnataka, India from Nov-2019 to Apr-2020. The experiment was carried out in cement cisterns, each with size of 20 m2 in open system. One control and two treatments of sugarcane bagasse substrates in triplicates were tried: No substrates, substrates and Substrate+feed. Here in called treatments T0, T1 and T2 respectively.
Sugarcane bagasse was soaked in water for two days in order to remove excess sugar and then sun dried properly. The bagasse was made into bundles of approximately 7.5 cm diameter and 1 m length by using nylon rope [17]. Bagasse bundles were hang vertically at rate of 5000 kg/ha in T1 and T2 with the regular intervals by using steel wire, across the pond by maintaining uniform distance of 20 cm between bagasse and tank inner surface as a free border zone and thus a total 20 bundles were tied in each pond.
Pond preparation, fish stocking and feeding
The experimental ponds were dried initially for a week to eradicate aquatic weeds and animals. Soil base of 15 cm was added and lime was applied at a rate of 250 kg/ha. On the following day water was filled in all tanks, and bagasse was installed in ponds according to experimental design.
After a week of liming, all ponds were fertilized with semidecomposed cow dung, urea and Single Super Phosphate (SSP) at a rate of 3000, 100, and 100 kg/ha respectively and thereafter manuring were continued fortnightly with the same dose till the end of experiment. The water level in the tanks was maintained at 90 cm ± 5 cm throughout the experimental period, and ponds were allowed for 7 days for the good growth of periphyton on bagasse bundles and planktons in water column.
Fingerlings of two indigenous fishes L. fimbriatus (35.97 ± 0.61 mm; 11.89 ± 0.67 g), B. carnaticus (13.39 ± 0.09 mm; 0.69 ± 0.02 g) and freshwater prawn larvae of M. rosennbergii (38.66 ± 0.88; 9.01 ± 0.28 g) were stocked at ratio of 3:3:4 respectively, with a total stocking density of 10,000/ha (i.e. 6, 6 and 8 individuals per pond respectively). Pelleted sinking feed of 30% protein were given to the treatment T0 and T2 at 5% of body weight in two weeks, there after it was reduced to 2% as per the body weight. The fishes and prawn were fed two times a day during the experimental period.
Water quality monitoring
Water samples were collected between 09.00 to 10.00 morning hours on weekly interval and analyzed for transparency (Sechhi disc), temperature (mercury thermometer) and pH (pH meter) and nutrients concentration [total ammonia-N (TAN), nitrite-N (NO2-N), nitrate-N (NO3-N) and phosphate-phosphorus (PO4-P)] by following standard protocols.
Growth performance and harvesting
Fishes and prawns were sampled fortnightly for regular assessment of growth and biomass. Substrates were removed before sampling and ponds were dragged to catch at least 50% of each species from the pond. The length and weight of each individual were recorded in each sampling. At the end of the experiment, fishes and prawns were harvested by draining the ponds. Species-wise SGR (% body weight/day), Survival (%), Gross yield (kg/ha) were calculated by following formulae:
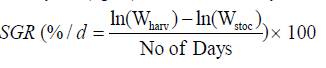
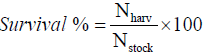
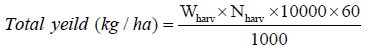
Proximate carcass composition
The carcass was analyzed for fish and prawn for crude protein, lipid, ash and moisture using the methods of AOAC (2016) at the beginning and end of the study. For this, muscle tissue of fish and prawn from each treatment were used.
Statistical analysis
All statistical analyses were done using SPSS 20.0. One-way ANOVA followed by Duncan’s multiple range test (P<0.05) was applied to find out the significance difference and homogeneity between the treatments.
Results
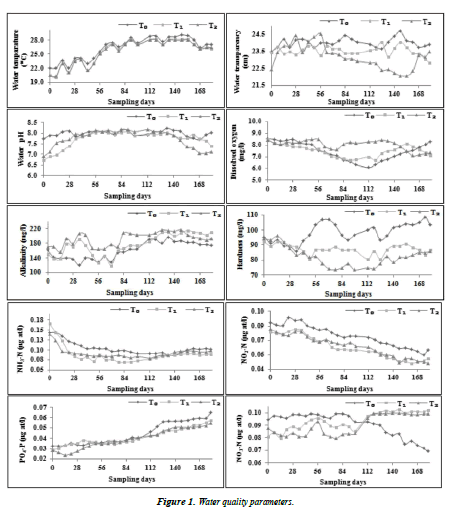
Water quality parameters
Physico-chemical characteristics of water recorded in the experiment are given in Table 1 and Figure 1. Except water temperature (20.03-29.07 °C), all other parameters like transparency (22.07-24.70 cm), pH (6.73-8.23), dissolved oxygen (6.07-8.53 mg/l) total alkalinity (120.00-215.67 mg/l) and hardness (73.33-108.33 mg/l), also nutrients such as NH3-N (0.068-0.167 mg/l), NO2-N (0.046-0.094 mg/l) NO3-N (0.069- 0.99 mg/l) and PO4-P (0.019-0.060 mg/l) showed significant difference between treatments and control ponds (P<0.05). DO concentration showed decreasing trend initially and the concentration was found high in T1 and T2 than T0. Low water pH and transparency observed in substrate treatments (T1 and T2) than the control. Total alkalinity concentration was found high in substrate treatments compared to control, whereas reverse trend observed for total hardness. The nutrients concentration NH3-N, NO2-N and PO4-P was found low in substrate, however NO3-N found high in substrate ponds.
Fish survival, growth and production
| Treatment | Temperature (°C) | Transparency (cm) | pH | DO (mg/l) | Tot. alkalinity (mg/l) | Tot. hardness (mg/l) | NH3-N(mg/l) | NO2-N(mg/l) | NO3-N(mg/l) | PO4-P(mg/l) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE |
Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| T0 | 26.30 | 0.45 | 24.00 | 0.05 | 8.00 | 0.03 | 7.50 | 0.15 | 160.60 | 4.70 |
98.40 | 1.25 | 0.104 | 0.003 | 0.075 | 0.002 | 0.09 | 0.002 | 0.04 | 0.002 |
| T1 | 25.60 | 0.49 | 23.50 | 0.06 | 7.70 | 0.07 | 7.50 | 0.09 | 177.40 | 5.93 |
87.20 | 0.61 | 0.089 | 0.003 | 0.063 | 0.002 | 0.093 | 0.001 | 0.036 | 0.002 |
| T2 | 25.50 | 0.48 | 23.10 | 0.14 | 7.70 | 0.08 | 8.00 | 0.09 | 191.70 | 3.79 |
82.10 | 1.26 | 0.092 | 0.002 | 0.064 | 0.002 | 0.09 | 0.002 | 0.035 | 0.002 |
Note: The values of different water quality parameters are mean ± SE of three ponds per treatment. |
||||||||||||||||||||
Table 1. Water quality parameters.
Labeo fimbiratus (141.17 g; 120.30 g), Barbodes carnaticus (98.07 g; 80.57 g) and Macrobrachium rosenbergii (73.95 g; 55.90 g) were showed good growth in T1 and T2 tanks respectively compared to T0 group (132.20 g; 86.73 g; 54.47 g for respective species). The highest survival observed in T2 (89.81%) compared to T1 (85.19%) and T0 (83.33%) and gross production (Kg/ha) also showed high in T2 (932.65) followed by T0 (728.89) and T1 (708.56). There is a significant difference (P<0.05) observed between the treatments. Fish and prawn growth, survival and production are given in Tables 2 and 3.
| Treatment | Species | Wstock (g) | Wharv (g) | Survival (%) |
SGR (%/d-1) |
Gross yield (Kg ha-1) |
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE |
|
|||||||||
| T0 | Lf | 10.67 | 0.90 | 132.20 | 1.54 | 83.33 | 9.62 | 1.39 | 0.04 | 988.95 | 34.62 |
|
|||||||
| Bc | 0.67 | 0.03 | 86.73 | 1.27 | 83.33 | 9.62 | 2.71 | 0.04 | 648.40 | 22.12 |
|
||||||||
| Mr | 8.33 | 0.26 | 54.47 | 2.08 | 83.33 | 4.17 | 1.04 | 0.03 | 545.90 | 14.24 |
|
||||||||
| T1 | Lf | 12.90 | 1.60 | 120.30 | 0.38 | 88.89 | 5.56 | 1.25 | 1.25 | 962.35 | 19.93 |
|
|||||||
| Bc | 0.67 | 0.03 | 80.57 | 0.83 | 83.33 | 9.62 | 2.67 | 0.03 | 605.35 | 25.05 |
|
||||||||
| Mr | 8.80 | 0.46 | 55.90 | 4.06 | 83.33 | 4.17 | 1.03 | 0.01 | 562.15 | 21.28 |
|
||||||||
| T2 | Lf | 11.90 | 0.95 | 141.17 | 0.66 | 94.44 | 5.56 | 1.38 | 0.05 | 1200.5 | 25.10 |
|
|||||||
| Bc | 0.73 | 0.03 | 98.07 | 1.16 | 88.89 | 5.56 | 2.72 | 0.03 | 784.60 | 16.78 |
|
||||||||
| Mr | 9.90 | 0.21 | 73.95 | 1.73 | 91.67 | 8.33 | 1.12 | 0.01 | 814.15 | 25.94 |
|
||||||||
Note: The values of growth parameters are mean ± SE of three ponds per treatment (Lf: L. fimbriatus; Bc: B. carnaticus; Mr: M. rosenbergii). |
|
|
|
||||||||||||||||
Table 2. Fish and prawn growth parameters.
| Species | Effect | df | MSS | F ratio | P value | |
|---|---|---|---|---|---|---|
| L fimbriatus | Between goups | 2 | 1235.351 | .727 | 0.485 | |
| Within groups | 114 | 1698.179 | ||||
| Total | 116 | |||||
| B. carnaticus | Between groups | 2 | 1120.381 | 1.363 | .260 | |
| Within groups | 114 | 822.044 | ||||
| Total | 116 | |||||
| M. rosenbergii | Between groups | 2 | 1501.566 | 3.672 | .028 | |
| Within groups | 114 | 408.908 | ||||
| Total | 116 | |||||
Note: df: Degree of Freedom; MSS: Mean Sum of Squares. |
||||||
Table 3. Analysis of variance (ANOVA) comparing the fish growth between treatments.
Carcass composition
The fishes and prawn showed high carcass protein content in T2 compared to T1 and T0. The protein content for L. fimbriatus (16.97 ± 0.07), B. carnaticus (21.79 ± 0.15) and M. rosenbergii (20.03 ± 0.13) was observed in T2 compared to T1 (16.90 ± 0.06; 21.58 ± 0.04; 19.89 ± 0.20) and T0 (16.29 ± 0.17; 20.63 ± 0.35; 19.02 ± 0.13) for respective species. Similarly, fat content (5.50 ± 0.40), (4.00 ± 0.01), (7.64 ± 0.08) and ash content (2.40 ± 0.34), (2.05 ± 0.13), (3.01 ± 0.06) for L. fimbriatus, B. carnaticus and M. rosenbergii respectively in T2 compared to T1 (5.29 ± 0.04; 4.13 ± 0.11; 7.36 ± 0.27), T0 (6.36 ± 0.10; 4.33 ± 0.09; 8.21 ± 0.18) for fat and T1 (2.65 ± 0.09; 2.20 ± 0.03; 3.12 ± 0.07), T0 (1.82 ± 0.07; 2.29 ± 0.02; 3.21 ± 0.07) for ash content. Moisture content in fish and prawn found low in T2 (75.09 ± 0.16), (69.57 ± 0.36), (69.18 ± 0.51) compared to T1 (74.36 ± 0.27; 70.52 ± 0.29; 71.05 ± 0.65) and T0 (74.89 ± 0.33; 70.72 ± 0.29; 70.36 ± 0.19) in respective species as shown in Table 4.
Treatment |
|
Protein (%) |
Fat (%) |
Ash (%) |
Moisture (%) |
|---|---|---|---|---|---|
L. fimbriatus |
|
||||
Initial |
|
13.20 ± 0.01 |
3.69 ± 0.01 |
1.40 ± 0.10 |
78.33 ± 0.04 |
T0 |
|
16.29 ± 0.17 |
6.36 ± 0.10 |
1.82 ± 0.07 |
74.89 ± 0.33 |
T1 |
|
16.90 ± 0.06 |
5.29 ± 0.04 |
2.65 ± 0.09 |
74.36 ± 0.27 |
T2 |
|
16.97 ± 0.07 |
5.50 ± 0.40 |
2.40 ± 0.34 |
75.09 ± 0.16 |
B. carnaticus |
|
||||
Initial |
|
18.72 ± 0.02 |
2.88 ± 0.06 |
1.58 ± 0.03 |
73.36 ± 0.03 |
T0 |
|
20.63 ± 0.35 |
4.33 ± 0.09 |
2.29 ± 0.02 |
70.72 ± 0.29 |
T1 |
|
21.58 ± 0.04 |
4.13 ± 0.11 |
2.20 ± 0.03 |
70.52 ± 0.29 |
T2 |
|
21.79 ± 0.15 |
4.00 ± 0.01 |
2.05 ± 0.13 |
69.57 ± 0.36 |
M. rosenbergii |
|
||||
Initial |
|
16.82 ± 0.01 |
5.21 ± 0.02 |
2.12 ± 0.02 |
76.55 ± 0.03 |
T0 |
|
19.02 ± 0.13 |
8.21 ± 0.18 |
3.21 ± 0.07 |
70.36 ± 0.19 |
T1 |
|
19.89 ± 0.20 |
7.36 ± 0.27 |
3.12 ± 0.07 |
71.05 ± 0.65 |
T2 |
|
20.03 ± 0.13 |
7.64 ± 0.08 |
3.01 ± 0.06 |
69.18 ± 0.51 |
Note: The values of carcass composition are mean ± SE of three ponds per treatment. |
|||||
Table 4. Carcass proximate composition of fish and prawn.
Discussion
Water quality parameters
Fish culture with combinations of cultured species, stocking densities and the quantity and quality of the nutrient inputs are highly influence the water quality parameters in the pond system [18]. In present experiment, low water temperature observed in substrate ponds, compared to control, this may be due to the shading effect by substrates. The transparency of water generally indicates the presence of natural food particles for fish and the productivity of the water body and the recommended range is 15-40 cm [19]. In the present study, transparency was in the recommended range. The low transparency in substrate ponds may be due to the presence of substrate which leads to leaching of nutrients and production of planktons. Water pH in T1 and T2 than T0 was mainly due to production of hydrogen ions because of high nitrification, due to nitrifying bacteria on substrate [20]. Banerjea stated that 5-7 ppm of dissolved oxygen of a water body is good for productivity and waters having dissolved oxygen below 5.0 ppm to be unproductive [21]. During the study period the DO concentration varied from 6.07–8.53 and initial DO depletion in all treatments may be due to the predominant heterotrophic food production which leads to the consumption of oxygen [22]. Also, the grazing activity induces water turbulence which helps to increase DO in water column [23]. The pH, dissolved oxygen observed during the study period was within suitable limit for tropical fish culture [24]. The high alkalinity value indicates higher nutrient turn over and productivity which were observed in ponds with substrates. It mainly caused by the effect of carbon dioxide released during decay of organic matter. The positive relationship was found between phytoplankton density and hardness, this might be the reason for high hardness in control group, as phytoplankton density was found more in control rather than substrate ponds [25].
Nitrifying bacteria are known to improve water quality by converting highly nitrogenous toxins such as ammonia and nitrite to nitrate [26]. The low concentration of ammonia-N and nitrite-N was observed in substrate-based treatments compared to the control may be due to the result of their nitrification by the microorganisms attached to the substrates, generating nitrate-N as the end product that helps in autotroph proliferation [27]. The preliminary increase of nitrite in all ponds attributes to oxidation of ammonia released from decomposition of manure added initially as reported [28]. The high concentration of nitrate in bagasse ponds was mainly due to leaching of nutrients from bagasse. Also, high densities of Chlorophyceae and Euglenophyceae, significantly increases nitrate level [29]. The phosphate phosphorus showed positive relationship (r=0.826) with alkalinity. Hoque revealed high phosphate-phosphorus content was mainly due to the periodical application of phosphate fertilizers [30]. However, increasing trend of PO4-P in substrate tanks was due to increase in the organic matter’s mineralization rate due to the higher heterotrophic bacterial biomass [31].
Fish survival, growth and production
Significant higher growth was observed in T2 for all three species. The highest final weight and survival of fish and prawn was due to positive effect of periphyton on water quality, as well as efficient nutrient cycling due to periodical fertilization. It was observed the high (P<0.05) growth parameters for L. fimbriatus in terms of final weight, weight gain (%) and biomass under Bagasse+Feed treatment [32]. The greater abundance of filamentous green algae and zooplankton might have caused higher growth of carnatic carp in substrate ponds. In addition to this, the presence of significantly high concentration of chlorophyll-a linked phytoplankton biomass in water and high load of nutrient rich organic debris in biofilm act as single cell protein source and also due to the low ammonia and nitrite nitrogenous waste [33, 34]. Periphyton algae on substrate help to enhance nitrate level in water which is utilized by autotrophs and thus increases the primary productivity in the culture system [7]. The high fish production in T2 was attributed to the microbial periphyton developed on substrate which acts as a food for zooplankton, fish and prawn. Likewise, periphyton substrates not only help to provide food for prawn but to minimize territoriality of freshwater prawn by providing extra shelter [35]. This might be the reason of significantly high survival and production of prawn in substrate ponds in current study. Similar results reported [36].
Carcass composition
At the end of experiment, fringed lipped carp, Carnatic carp and freshwater prawn from substrate ponds showed comparatively high protein, fat and ash percentage than that of initial values, whereas moisture found low. The findings were similar and decreased carcass moisture with increasing dietary protein was reported [37-39]. The higher protein and fat content in the carcass of the treated fish indicates that there was favorable effect of biofilm on the carcass composition of fish and prawn. The biodegradable substrates like bagasse having more fibre and rough surface area, which favored higher production of heterotrophic bacterial population along with high quantities of colonized phytoplankton and zooplankton which in turn helps for better growth and nutrition of fishes.
The results of the experiment showed that periphyton significantly improved water quality by lowering concentration of toxic ammonia and nitrite. Also, periphyton along with supplementary feed treatment helps to increase the protein percentage in the fish and prawn. Sugarcane bagasse can be used as substrate in the cultural systems for enhancement of health and growth of the fish with increasing the production which helps for sustainable, environment-friendly and cost effective practice for resource poor farmers.
Conclusion
The results of the experiment showed that periphyton significantly improved water quality by lowering concentration of toxic ammonia and nitrite. Also, periphyton along with supplementary feed treatment helps to increase the protein percentage in the fish and prawn. Sugarcane bagasse can be used as substrate in the cultural systems for enhancement of health and growth of the fish with increasing the production which helps for sustainable, environment-friendly and cost effective practice for resource poor farmers.
References
- Green BW, Schrader K, Perschbacher PW, et al. Effect of stocking biomass on solids, phytoplankton communities, common off-flavors, and production parameters in a channel catfish biofloc technology production system. Aquac Res. 2014; 45 (9):1442-58.
- Avnimelech Y. C/N ratio as a control element in aquaculture systems. Aquaculture. 1999; 176: 227-35
- Schroeder GL. Autotrophic and heterotrophic production of microorganisms in intensely-manured fish ponds and related fish yields. Aquaculture. 1978; 14: 303-25.
- Shilta M, Christina L, Bindu P, et al. Biofilm developed on plant substrates enhances growth and survival of post larvae of Macrobrachium rosenbergii. Fish Technol. 2020; 57: 88-97.
- Asaduzzaman M, Wahab MA, Verdegem MCJ, et al. Effects of addition of tilapia Oreochromis niloticus and substrates for periphyton developments on pond ecology and production in C/N-controlled freshwater prawn Macrobrachium rosenbergii farming systems. Aquaculture. 2008; 287: 371-80.
- Azim ME, Little DC. Intensifying aquaculture production through new approaches to manipulating natural food. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour. 2006; 1: 62.
- Thompson FL, Abreu PC, Wasielesky WJ, et al. Importance of biofilm for water quality and nourishment in intensive shrimp culture. Aquaculture. 2002; 203: 263-78.
- Tidwell JH, Coyle SD, Vanarnum A, et al. Production response of freshwater prawns Macrobrachium rosenbergii to increasing amounts of artificial substrate in ponds. J World Aquac Soc. 2000; 31: 452-58.
- Keshavanath P, Gangadhar B, Ramesh TJ, et al. Performance of indigenous carps, Tor khudree and Labeo fimbriatus in fed and non-fed tanks with different bamboo substrate densities. Aquaculture. 2002; 213: 207-18.
- Uddin MS, Azim ME, Wahab MA, et al. Effects of substrate addition and supplemental feeding on plankton composition and production in tilapia (Oreochromis niloticus) and freshwater prawn (Macrobrachium rosenbergii) polyculture. Aquaculture. 2009; 297: 99-105.
- Khatoon H, Yusoff F, Banerjee S, et al. Formation of periphyton biofilm and subsequent bio-fouling on different substrates in nutrient enriched brackish water shrimp ponds. Aquaculture. 2007; 273: 470-477.
- Shahabuddin AM, OO MT, Thakur DP, et al. Study about the effect of rice straw mat on water quality parameters, plankton production and mitigation of clay turbidity in earthen fish ponds. World J Medical Sci. 2012; 4: 577-85.
- Gangadhar B, Keshavanath P. Growth performance of rohu, Labeo rohita (Ham.) in tanks provided with different levels of sugarcane bagasse as periphyton substrate. Indian J Fish. 2012; 59: 77-82.
- Shilta MT, Chadha NK, Pandey PK, et al. Effect of biofilm on water quality and growth of Etroplus suratensis (Bloch, 1790). Aquac Int. 2016; 24: 661-74.
- Sayed AFM. Tilapia Culture. CAB International. 2006; p: 304.
- Mridula RM, Manissery JK, Keshavanath P, et al. Water quality, biofilm production and growth of fringed lipped carp (Labeofimbriatus) in tanks provided with two solid substrates. Bioresour Technol. 2003; 87: 263-67.
- Keshavanath P, Gangadhar B, Ramesh TJ, et al. Use of artificial substrates to enhance production of freshwater herbivorous fish in pond culture. Aquac Res. 2001; 32(3): 189-97.
- Milstein A. Water quality and freshwater ?sh culture intensi?cation: the Israeli example. Aquaculture and Fisheries Management. 1993; 24: 715-24.
- Boyd CE (1982) Water quality management for pond ?sh culture. Elsevier, Amsterdam.
- Henriksen K, Kemp WM. Nitrification in estuarine and coastal marine sediments: Nitrogen cycling in coastal marine environments. Wiley.1988; pp: 207-49.
- Banerjea SM. Water quality and soil condition of fish ponds in some states of India in relation to fish production. Indian J Fish. 1967; 14: 115-44.
- Moriarty DJW. The role of microorganisms in aquaculture ponds. Aquaculture.1997; 151: 333-49.
- Moyle JB. Some indices of lake productivity. Trans Am. 1949; 76(1): 322-334.
- Boyd C, Zimmermann S. Grow-out systems–water quality and soil management. In: Freshwater prawn culture: the farming of Macrobrachium rosenbergii. Blackwell Science, Oxford. 2000; pp: 221–38.
- Jasmine S, Islam R, Rahman M, et al. Plankton production in relation to water quality parameters in lentic and lotic water bodies during post-monsoon season in the northwestern Bangladesh. J Agric Environ. 2013; 2: 270-76.
- Viau, VE, Marciano A, Iriel A, et al. (2006). Assessment of a biofilm-based culture system within zero water exchange on water quality and on survival and growth of the freshwater shrimp Neocaridina heteropoda. Aquaculture Research Aquac Res. 2006; 47:2528-42.
- Pan J, Yuan Y, Zhang L, et al. Nitrogen removal in subsurface wastewater infiltration systems with and without intermittent aeration. Ecol Eng. 2016; 94: 471-77.
- James PSB. Overview of oyster culture: Present status and future prospects. In: Oyster culture - status and prospects (ed) Nayar, N. K. and Mahadevan, S. Bulletin of Central Marine Fisheries Research Institute.1987 38: pp: 75-8.
- Sipauba TLH, Donadon ARV, Milan RN, et al. Water quality and plankton populations in an earthen polyculture pond. Braz J Biol. 2011; 71(4): 845-55.
- Hoque MI, Rahman AKMF, Mansur MA, et al. Effects of periphyton on monoculture of Puntius gonionotus. IJARIT. 2018; 8(2): 13-23.
- Baloi M, Arantes R, Schveitzer R, et al. Performance of Pacific white shrimp Litopenaeus vannamei raised in biofloc systems with varying levels of light exposure. Aquac Eng. 2013; 52: 39-44.
- Gangadhar B, Sridhar N, Latha U, et al. Growth performance and digestive enzyme activities of fringe-lipped carp Labeofimbriatus (Bloch, 1795) in periphyton based nursery rearing system. Indian J. Fish. 2016; 63(1): 125-31.
- Anupama, Ravindra PP. Value-added food: single cell protein. Biotechnol.2000; 18:459-79.
- Azim ME, Verdegem MCJ, Rahman MM, et al. Evaluation of polyculture with Indian major carps in periphyton based ponds. Aquaculture.2002; 213: 131–49.
- Ahsan ME, Sharker MR, Alam MA, et al. Effects of addition of tilapia and periphyton Substrates on water quality and abundance of plankton in freshwater prawn culture ponds. Int J Sci Technol Res. 2014; 3(2): 272-78.
- Hasan MN, Rahman MS, Hosen MF, et al. Effects of addition of tilapia on the abundance of periphyton in freshwater prawn culture ponds with periphyton substrates. J Bangladesh Agril Univ. 2012; 10(2): 313-24
- Dharmaraj M, Manissery JK, Keshavanath P, et al. Effects of biodegradable substrate, sugarcane bagasse and supplemental feed on growth and production of fringed-lipped peninsula carp, Labeo fimbriatus (Bloch). Acta Ichthyol Piscat. 2002; 32: 137-44.
- Bharti V, Pandey PK, Vennila A, et al. Water quality, survival and growth performance of Cirrhinus mrigala (Hamilton 1822) in substrate based tanks. Asian Fish Soc. 2016; 29: 137-50.
- Jana SN, Garg SK, Arasu ART, et al. Use of additional substrate to enhance growth performance of milkfish, Chanos chanos (Forsskal) in inland saline groundwater ponds. J Appl Aquac. 2006; 18: 1-20.