Research Article - Journal of Gastroenterology and Digestive Diseases (2018) Volume 3, Issue 3
Evaluation of Golgi protein 73 as a prognostic marker after loco-regional ablation of HCC in Hepatitis C cirrhotic patients.
Nahla Khalaf1*, Mahmoud El-Kadeem1, Amany Abo Elenein2, Gamal Kasem1 and Elsayed Wasfi1
1Faculty of Medicine, Department of Tropical Medicine & Infectious Diseases, Tanta University, Tanta, Egypt
2Faculty of Medicine, Department of Clinical Pathology, Tanta University, Tanta, Egypt
- *Corresponding Author:
- Nahla Khalaf MD
Faculty of Medicine
Department of Tropical Medicine & Infectious Diseases
Tanta University, Tanta, Egypt
Tel: +201099454124
E-mail: dr_nohnoh86@yahoo.com
Accepted on November 05, 2018
Citation: Khalafa N, El-Kadeema M, Elenein AA, et al. Evaluation of Golgi protein 73 as a prognostic marker after Locoregional ablation of HCC in Hepatitis C cirrhotic patients. J Gastroenterol Dig Dis.2018;3(3):49-55.
Abstract
Background & Aim: Hepatocellular carcinoma is a global health problem. Its diagnosis depends mainly on abdominal ultrasound and serum alpha-fetoprotein. Golgi protein 73 is a type II membrane protein upregulated in hepatocytes. Serum Golgi protein 73 could be a promising biomarker for diagnosis and prognosis of hepatocellular carcinoma. The aim was to assess serum Golgi protein 73 as a marker for follow up of hepatocellular carcinoma patients after either radiofrequency or microwave ablation in comparison to alpha-fetoprotein.
Methods: This study was performed on 80 subjects (60 chronic hepatitis C and liver cirhosis, and 20 controls). They were divided into three groups; Group I: included 30 patients with hepatocellular carcinoma, Group II: included 30 patients without hepatocellular carcinoma, and Group III: included 20 healthy individuals as control ones. Golgi protein 73 was measured in all subjects and followed up in group I one, 3, and 6 months after locoregional ablation.
Results: Both alpha-fetoprotein and Golgi protein 73 were significantly higher in group I than either group II or group III. Alpha-fetoprotein had a cut off value >20 ng/mL (sensitivity 86.67%, specificity 84%). Golgi protein 73 level Golgi protein 73 had a cut off value >79.2 ng/mL (sensitivity 96.67%, specificity 96%). Both markers were decreasing after ablation in different periods of follow up. Golgi protein 73 was correlated positively with alpha-fetoprotein, ALT, and AST.
Conclusion: Golgi protein 73 is a useful marker in diagnosis of hepatocellular carcinoma, also in follow up after locoregional therapy to detect early recurrence.
Keywords
Hepatocellular carcinoma, Alpha-fetoprotein, Golgi protein 73, Prognosis.
Introduction
Hepatocellular carcinoma (HCC) is a widespread malignant liver tumor, and it is the third cancer causing death all over the world [1]. The major risk factors for HCC are chronic hepatitis B virus (HBV) hepatitis C virus (HCV), and liver cirrhosis on top of them [2]. The five-year survival rate of HCC patients is low (10.1%) because many cases are diagnosed in advanced stages and high rate of recurrence and metastasis [3,4]. HCC is often detected by ultrasonography and serum alpha-fetoprotein (AFP). Despite AFP has high specificity (80-94%), its sensitivity is only (41-65%) [5]. Other serum biomarkers have emerged to improve the efficiency of early diagnosis of HCC. One of them is Golgi protein 73 (GP73) [6].
GP73 is a type II Golgi membrane protein. Its molecular weight is 73 kDa. In the normal liver tissue, Gp73 is mainly expressed by biliary epithelial cells. However, its expression in chronic liver diseases, either viral or not, liver cirrhosis and HCC is mainly hepatocytes [7,8]. GP73 over-expression is induced in inflammatory process, specifically by inflammatory cytokines such as IL-6, possibly in response to chronic active viral infection [9]. The elevated levels of GP73 expression identified in HCC may be due abnormal core fucosylation to GP73 by α1, 6-fucosyltransferase enzyme (Fut) that increases in HCC. Fut overexpression causes dramatically enhanced expression of GP73 at the level of transcription. However, the precise mechanism of GP73 elevation in HCC is still unclear [10,11]. Recent studies detected the efficacy of biomarker GP73 in the early diagnosis of HCC with high sensitivity and specificity in comparison to AFP [12,13]. However, serum GP73 levels were detected to be higher in patients with liver cirrhosis than those with HCC in other studies making its diagnostic value controversial [14,15]. On the other hand, GP 73 prognostic value was detected in some studies. An association between high levels of GP 73 and tumor aggression, metastasis, poor overall survival was detected, while the exact mechanism was unknown [16,17]. This study aimed to assess serum Golgi protein 73 as a marker for follow up of HCC patients who underwent either radiofrequency or microwave ablation in comparison to AFP levels.
Methods
This is a prospective cohort study performed on 80 subjects from the outpatient clinics and inpatients of Tropical Medicine and Infectious Diseases Department in Tanta University Hospitals within one-year period between February 2015 till February 2016. The study protocol was approved by Tanta University Institutional Review Board. Written informed consent was obtained from every participant before the beginning of the study.
Demographic data and clinical information were obtained, and a blood sample was collected from every subject under complete aseptic precautions. The subjects of the study were classified into three groups: Group I: included 30 hepatitis C cirrhotic patients with HCC. Group II: included 30 hepatitis C cirrhotic patients without HCC. Group III: included 20 healthy individuals as a control group.
Patients who had HCV infection, and aged 18 years or more, and patients who had HCC suitable for a locoregional therapy (radiofrequency ablation (RFA), or microwave ablation (MVA)) were included in the study. However, patients who refused to participate in the study, patients whose HCC not suitable for a locoregional therapy, patients with metastasizing HCC, patients who had HCC on top of risk factors other than chronic HCV infection, or patients with extrahepatic malignancies were excluded.
HCC was Diagnosed by either two imaging modalities (ultrasound, computed tomography or magnetic resonance imaging) showing lesion with arterial enhancement and venous washout, or histopathology when imagining was not conclusive. The diagnosis of liver cirrhosis was based on liver histology or clinical, laboratory and imaging evidence of hepatic decompensation or portal hypertension.
Data collection
Informed consent was taken from every patient after explaining the whole procedure. Demographic data were collected. History taking, clinical examination, laboratory investigations (including liver, renal functions, complete blood count, alpha-fetoprotein), and radiological investigations including abdomino-pelvic ultrasound and triphasic abdominal CT with contrast were done. Staging of HCC according to BCLC and severity of liver disease (according to Child-Pugh score) were evaluated. The first group underwent loco regional therapy by either microwave ablation or RFA and followed up over a period of six months by triphasic CT scan, AFP & GP 73.
Determination of serum level of GP 73
Quantitative measurement of human GP73 was based on a sandwich ELISA technique for (ELISA kit provided by Sun Red biotechnology Co. (Planegg, Germany)). Samples were diluted 20-fold in phosphate-buffered saline. Then; 100 μl of diluted serum sample was added to the microtiter plate well pre-coated with an antibody specific to GP73 and incubated at 37˚C for 2 h. Anti-IgG conjugated with biotin was added to each well and incubated for 1 h. The plate was washed three times with buffer. Avidin conjugated to horseradish peroxidase was added to each well and incubated for 30 min. Washing was done five times, 90 μl of tetramethylbenzidine substrate solution was added to each well and incubated in the dark for 15 min. The enzyme-substrate reaction was then terminated by the addition of 50 μl of sulfuric acid solution. Color change was measured spectrophotometrically at a wavelength of 450 nm. Concentration of sGP73 in the samples was determined by comparing the optical density of the samples to the standard curve [18].
Loco-regional ablation of HCC
Ablation was directed to the entire focal lesion and 1 cm of tumor free margin of normal liver. The procedure was done in a special sterilized unit containing the ultrasound machine (Siemens, Toshiba). First of all, patients were fasting 6 hours. Sterilization of skin was made using betadine and alcohol. Local anesthesia was performed by 10 ml of 2% xylocaine. It was along the needle track from the entry site on the skin to the liver capsule.
RFA was made using The RITA ® Model 1500x RF,” produced by Angio-Dynamics, USA”. As an RFA session began, a hyperechoic focus developed around the uninsulated portion of the electrode. This was attributed to tissue vaporization and cavitations. The area of echogenicity was round; most often progressively increased in size over the course of ablation and generally enveloped the entire tumor with variable extensions in the surrounding liver by the end of the treatment. When the time was over, the generator automatically went into cool down mode for 30 seconds (5minutes on the generator display), when the Cool Down was complete, the temperatures from all leads had to be above 70°C, if not ablation would continue for another 5 minutes at target temperature. In all cases, tract ablation was done before removal of the needle.
As regards MVA the microwave needle (AMICA™ - MW Ablation System) was inserted deep in the lesion avoiding big vessels and surrounding viscera. Ablation was done using 80 Watt for 10 minutes to achieve volume ablation of 25-40% according to safety. When ablation was completed needle track ablation was done to avoid post procedural bleeding. Strong IV analgesics were given to eradicate the pain as pethidine hydrochloride 50 mg or tramadol and intravenous antiemetic was given if needed. All patients were observed clinically for 2-3 hours. Prophylactic antibiotic was given as, amoxicillin-clavulanic acid or ceftazidime, and metronidazole 1 hour before procedure and continued for 5 days.
Statistical analysis
SPSS (Statistical Package for the Social Sciences) (V.19 SPSS Inc, Chicago, IL, USA) program was used for statistical analysis. Mean and standard deviation were calculated. One way analysis of variance (ANOVA) was used to analyze normally distributed quantitative data. Pearson Chi- square test, and Fisher,s Exact test were used to analyze of the qualitative data. Pearson correlation test was done to detect relation between two quantitative variables. Significance was established as p<0.05. Receiver operating characteristic (ROC) curve was plotted and cut off values of both GP 73, and AFP was calculated with sensitivity, specificity, positive predictive value, and negative predictive value [19].
Results
Demographic data was demonstrated in the Table 1. As regards laboratory data, there were significant differences in liver function tests between three groups with the highest mean in group II. However, serum AFP, and GP73 were significantly higher in group I than other groups, and higher in group II than control group (Tables 1 and 2). In group I, a significant positive correlation was detected between AFP and GP 73 (r= 0.608) (p-value = 0.001). In addition; both GP73, AFP were positively correlated with AST (r= 0.521) (p-value = 0.003), (r= 0.433) (p-value = 0.017) respectively, and GP 73 showed significant positive correlation with ALT (r= 0.429) (p-value = 0.018) (Table 3).
| Group | HCC group (n=30) | HCV group (n=30) | Control group (n=20) | P – value |
|---|---|---|---|---|
| Age (mean + SD) | 57.067 ± 7.794 | 54.967 ± 8.294 | 52.400 ± 10.002 | 0.174 |
| Gender (N (%))# | ||||
| Male / Female | 23 (76.67) / 7 (23.33) | 21 (70) / 9 (30) | 12 (60%) / 8 (40%) | 0.452 |
| Laboratory data (mean + SD) | ||||
| Total bilirubin (mg/dl) | 1.315 ± 1.206 | 2.877 ± 3.016 | 0.9 ± 0.172 | 0.001* |
| Direct bilirubin (mg/dl) | 0.590 ± 0.745 | 1.547 ± 2.600 | 0.162 ± 0.048 | 0.012* |
| ALT (IU/L) | 43.133 ± 0.240 | 53.26 ± 33.05 | 21.60 ± 11.34 | 0.001* |
| AST (IU/L) | 60.333 ± 39.082 | 80.83 ± 65.78 | 25.800 ± 12.870 | 0.001* |
| Albumin (gm/dl) | 3.647 ± 0.521 | 2.897 ± 0.596 | 3.860 ± 0.272 | <0.001* |
| INR | 1.204 ± 0.105 | 1.364 ± 0.338 | 1.099 ± 0.092 | <0.001* |
| Alpha-fetoprotein (ng/ml) | 105.071 ± 87.646 | 25.583 ± 35.735 | 6.920 ± 2.938 | <0.001* |
| Golgi protein 73 (ng/ml) | 214.283 ± 112.61 | 22.132 ± 20.456 | 17.982 ± 16.614 | <0.001* |
| Hb. (gm/dl) | 12.136 ± 1.636 | 11.130 ± 1.927 | 12.160 ± 1.023 | 0.031* |
| Platelets × 103/cmm | 105.500 ± 63.602 | 133.700 ± 59.55 | 305.60 ± 105.38 | <0.001* |
| WBCs × 103/cmm | 5.690 ± 2.889 | 5.870 ± 2.487 | 8.760 ± 2.446 | <0.001* |
| Child score (N (%))# | ||||
| A | 25 (83.33) | 7 (23.33) | ---- | <0.001* |
| B | 5 (16.67) | 17 (56.67) | ||
| C | 0 (0) | 6 (20) | ||
*Significant. #Chi-square test. Other tests are one way ANOVA.
Table 1. Comparison between demographic and laboratory data of the three groups including alpha-fetoprotein and Golgi protein 73.
| Variables | TUKEY'S Test | ||
|---|---|---|---|
| Group I & II | Group I & III | Group II & III | |
| Total bilirubin (mg/dl) | 0.009* | 0.752 | 0.003* |
| Direct bilirubin (mg/dl) | 0.072 | 0.646 | 0.014* |
| ALT (IU/L) | 0.347 | 0.026* | 0.001* |
| AST (IU/L) | 0.221 | 0.036* | <0.001* |
| Albumin (gm/dl) | <0.001* | 0.313 | <0.001* |
| INR | 0.018* | 0.232 | <0.001* |
| Alpha-fetoprotein (ng/ml) | <0.001* | <0.001* | 0.509 |
| Golgi protein 73 (ng/ml) | <0.001* | <0.001* | 0.977 |
| Hb. (gm/dl) | 0.050* | 0.999 | 0.08 |
| Platelets × 103/cmm | 0.316 | <0.001* | <0.001* |
| WBCs × 103/cmm | 0.962 | <0.001* | <0.001* |
*Significant
Table 2. Comparison between laboratory data of each two groups including alpha-fetoprotein and Golgi protein 73.
| Parameters | Group I | Group II | ||||||
|---|---|---|---|---|---|---|---|---|
| Golgi protein 73 | Alpha-fetoprotein | Golgi protein 73 | Alpha-fetoprotein | |||||
| r | P-value | r | p-value | r | P-value | r | p-value | |
| Alpha-fetoprotein | 0.608 | <0.001* | - | -- | 0.004 | 0.983 | - | - |
| Golgi protein 73 | - | - | 0.608 | <0.001* | - | - | 0.004 | 0.983 |
| Age | 0.086 | 0.651 | 0.214 | 0.256 | 0.138 | 0.467 | 0.129 | 0.497 |
| Total bilirubin | 0.248 | 0.186 | 0.136 | 0.474 | 0.08 | 0.674 | 0.283 | 0.13 |
| Direct bilirubin | 0.206 | 0.275 | 0.045 | 0.813 | 0.09 | 0.636 | 0.213 | 0.26 |
| ALT | 0.429 | 0.018* | 0.288 | 0.123 | 0.095 | 0.618 | 0.152 | 0.423 |
| AST | 0.521 | 0.003* | 0.433 | 0.017* | 0.151 | 0.426 | 0.055 | 0.773 |
| Albumin | -0.185 | 0.327 | -0.33 | 0.075 | -0.566 | 0.001* | -0.033 | 0.863 |
| INR | -0.131 | 0.49 | -0.18 | 0.341 | -0.257 | 0.17 | -0.068 | 0.721 |
| HB | -0.086 | 0.651 | -0.238 | 0.205 | -0.245 | 0.192 | -0.076 | 0.69 |
| Platelets | -0.21 | 0.265 | -0.165 | 0.384 | -0.196 | 0.3 | -0.214 | 0.256 |
| WBCs | 0.129 | 0.497 | 0.135 | 0.477 | 0.214 | 0.256 | 0.697 | <0.001* |
*Significant
Table 3. Pearson correlation between Golgi protein 73, Alpha-fetoprotein levels and other parameters.
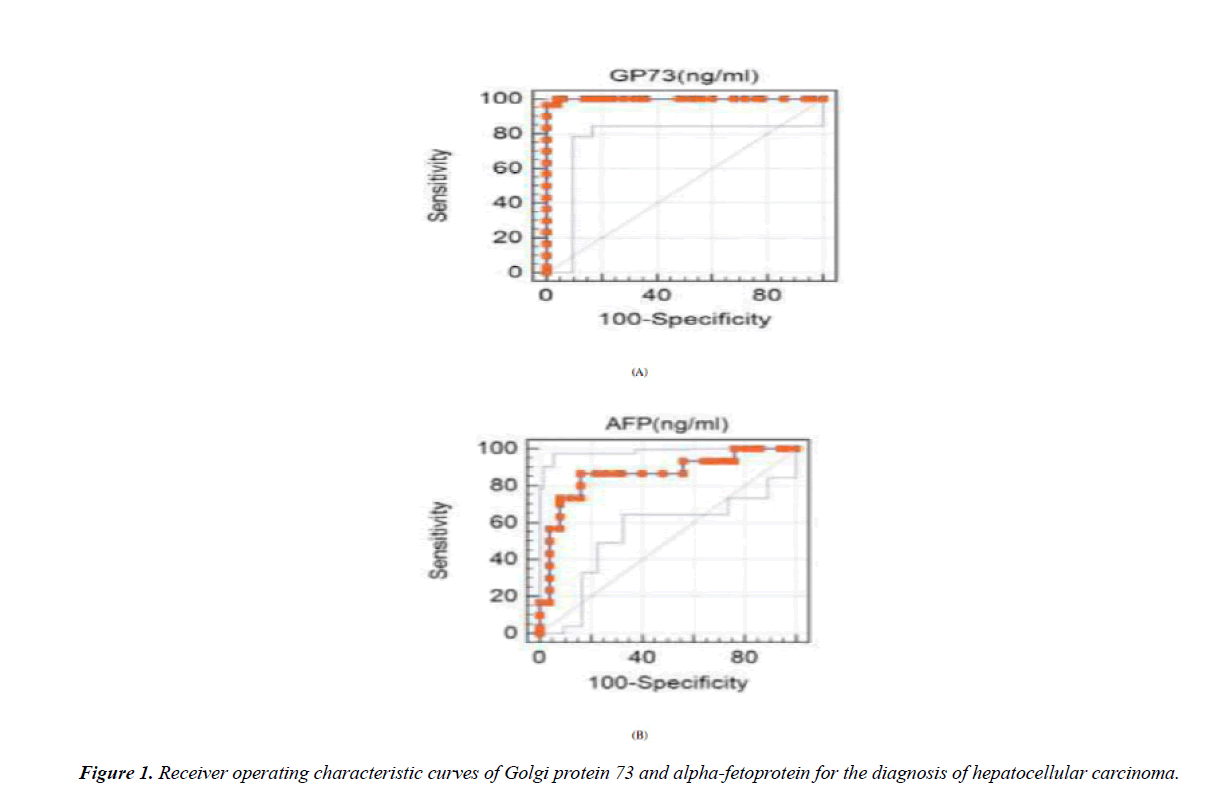
Receiver operating characteristic (ROC) curve was plotted to discriminate between HCC group and cirrhotic liver group. Cut – off values for AFP, and GP 73 were calculated (with sensitivity, specificity, and positive predictive value). GP 73: (cut off value 79.2 ng/mL, sensitivity 96.67%, specificity 96%, and positive predictive value 93.5%), and AFP level: (cut off value 20 ng/mL, sensitivity 86.67%, specificity 84%, and positive predictive; value 76.5%) (Table 4 and Figure 1).
| Parameters | Cut off | Sensitivity | Specificity | Positive | Negative |
|---|---|---|---|---|---|
| (%) | (%) | Predictive Value | Predictive Value | ||
| Golgi protein 73 (ng/ml) | >79.2 | 96.97 | 96 | 93.5 | 98 |
| Alpha-fetoprotein (ng/ml) | >20 | 86.67 | 84 | 76.5 | 91.3 |
Table 4. Diagnostic performance of Golgi protein 73 and Alpha-fetoprotein for discrimination of HCC from chronic liver disease cases.
In comparison between basal levels of AFP, GP73, and their levels after locoregional ablation, significant decrease was detected in every follow up either 1, 3, or 6 months. Mean baseline AFP was (105.071 ± 87.646), after one month it was (72.133 ± 58.033) (p-value = 0.001), after three months it was (60.960 ± 41.189) (p-value = 0.002), and after six months it was (53.004 ± 36.147) (p-value = 0.001). As regards GP 73, mean baseline was (214.283 ± 112.609), after one month it was (79.771 ± 63.137) (p-value < 0.001), after three months it was (36.813 ± 35.554) (p-value < 0.001), and after six months it was (17.979 ± 17.136) (p-value < 0.001) (Table 5).
| Parameters | Alpha-fetoprotein (ng/ml) | Golgi protein 73 (ng/ml) | ||||
|---|---|---|---|---|---|---|
| Baseline (mean + SD) | 105.071 ± 87.646 | 214.283 ± 112.609 | ||||
| (Mean + SD) | Paired t - test | p-value | (Mean + SD) | Paired t - test | p-value | |
| Follow up | ||||||
| One month | 72.13 ± 58.03 | 3.81 | 0.001* | 79.77 ± 63.14 | 6.629 | <0.001* |
| Three months | 60.96 ± 41.19 | 3.411 | 0.002* | 36.81 ± 35.55 | 9.035 | <0.001* |
| Six months | 53.004 ± 36.15 | 3.88 | 0.001* | 17.98 ± 17.14 | 10.105 | <0.001* |
*Significant
Table 5. Comparison of Golgi protein 73 and Alpha-fetoprotein levels before loco-regional ablation and 1, 3, 6 months after ablation.
Discussion
Alpha-fetoprotein (AFP) is an important marker for early diagnosis and follow up of liver cancer progression. However, high AFP levels can be detected in liver cirrhosis or exacerbations of chronic hepatitis and about 30-40% of liver cancers are negative for AFP expression [20]. Prospective studies analyzing the value of AFP in HCC surveillance reported sensitivities to be 39-64%, specificities to be 76-91% and positive predictive values to be 9-32% [21,22]. So that, there is a question about the clinical value of AFP, and novel serum markers for liver cancer are being actively sought in current research [23]. Golgi protein 73 is usually expressed in human epithelial cells, not in normal hepatocytes. However, it shows high expression in hepatocytes in liver diseases, especially in HCC patients [24]. The great interest has been directed towards serum GP73 because of its potent role in the diagnosis of HCC. Western blotting, immunoblotting, and ELISA are three major methods used to assay GP73 [25].
In this study, AFP level was significantly higher in group I (HCC group) than group II (HCV induced cirrhosis) and higher in group I than group III (control), while no significant difference was found between group II & III. Lok et al. [26]; reported an increase in AFP levels before detecting any suspicious liver nodule by using ultrasound in 6 of 39 patients with HCC. This indicates that AFP measurement and ultrasound can be complementary.
In a study made by Yousuf et al. [27]; AFP was not detected to be elevated in all HCC patients. In our study, AFP was found normal, less than 20 ng/mL, in 4 cases of group I in the pretreatment period; meanwhile, GP73 was high. This explained the usefulness of GP73 assay in AFP negative HCC. This finding was similar to Zhang et al. [28]; who detected that the commonly used AFP cut-off (20 ng/ mL) had unsatisfactory sensitivity in the detection of early-stage HCC; and up to 50% of patients with AFP level below 20 ng/mL had HCC. Another study stated that 66% of patients with AFP-negative HCC were positive for Golgi protein 73 (GP73) [29].
In the present study, there was high statistically significant difference between HCC group and control group regarding GP73 (P<0.001), also there was high statistically significant difference between HCC group and liver cirrhosis group regarding GP73 (P<0.001). This was supported by Fathy et al. [30], and Marrero et al. [31] who reported that sGP73 levels increased significantly in patients with HCC on top of chronic HCV in comparison to cirrhotic controls. However, another study suggested that sGP73 might not be suitable as a general marker of HCC but might be useful as a marker of HCV-related HCC. These findings were in doubt due to the relatively small number of total and HCV-related HCC cases [32].
In this study, the cut-off value 79.2 ng/mL of sGP73 had sensitivity 96.67% and specificity 96% in comparison to cut-off value of AFP (20 ng/mL) which sensitivity and specificity were 86.67% and 84% respectively. This was in agreement with the study made by Khalil et al. [33] who concluded that GP73, as a marker for HCC, had higher accuracy, sensitivity, and specificity than AFP. Sensitivity and specificity of SGP73 for HCC were 97.1 and 85.7% respectively compared with 57 and 55.6% for AFP.
In agreement with our results, multi-center study in 2008 compared serum GP73 and AFP in 4217 subjects as regards sensitivity and specificity in patients at risk for HCC development. The results were: the sensitivity and specificity of serum GP73 for HCC were 74.6% and 97.4%, compared with 58.2 and 85.3% for AFP (P<0.001) using 35 ng/mL as a cut-off value. The GP73 level was significantly high in patients with HCC in comparison to healthy controls (14.7 vs. 1.2, P <0.001). GP73 decreased after surgical resection of HCC lesions and increased with tumor recurrence [6].
In this study, significant positive correlation was detected between GP 73 in HCC group, and some other parameters (AFP, AST, and ALT). This was similar to another study which revealed significant correlation between serum GP73 level and prognostic markers of liver cirrhosis (AST, ALT, serum albumin, and child score) [34]. However, Hou et al. [11]; stated that there were no correlations between serum GP73 levels and the additional parameters, including tumor size and grading.
As regards follow up, significant decrease in both AFP and GP 73 after locoregional ablation in all stations of follow up (after one month, three months, and six months). These findings mean that GP 73 has prognostic role in patients with HCC. In agreement with the present study, another study showed that AFP, and GP-73 levels all sharply decreased after RFA, which indicated that these tumor markers could reflect the tumor burden and demonstrate the efficacy of RFA [35]. In a similar study measured serum GP73 before and after transarterial chemoembolization of HCC, levels decreased 7 days post-intervention in comparison to those recorded prior to treatment. GP73 serum levels (30 days post-intervention) were significantly higher in patients with disease progression than those of patients in remission [36].
On the other hand, it was detected that a high level of sGP73 was associated with aggressive clinicopathological features of HCC and poor overall survival. Also, a high level of sGP73 in patients with resectable HCC was associated with significantly decreased disease-free survival and overall survival compared with a low level of sGP73. So that, sGP73 may be unsuitable as a diagnostic marker for the early detection of HCC; however, it can be an independent negative prognostic marker [37].
Conclusion
In conclusion, GP73 is an accurate serum marker for the detection of HCC with higher sensitivity and specificity than AFP, and it is a useful prognostic marker also for follow up of HCC after loco-regional therapy. However, increase the sample size and extension of the follow-up time may be needed in future researches.
Conflicts of Interest
There are no conflicts of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893-2917.
- Shariff MI, Cox IJ, Gomaa AI, et al. Hepatocellular carcinoma: Current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol. 2009;3(4):353-67.
- Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003-2005: Apopulation-based study. Int J Cancer. 2015;136 8):1921-30.
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-27.
- Debruyne EN, Delanghe JR. Diagnosing and monitoring hepatocellular carcinoma with alpha-fetoprotein: New aspects and applications. Clin Chim Acta 2008;395(1-2):19- 26.
- Mao Y, Yang H, Xu H, et al. Golgi protein 73(GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut 2010;59(12):1687-93.
- Kladney RD, Cui X, Bulla GA, et al. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology 2002;35(6):1431-40.
- Iftikhar R, Kladney RD, Havlioglu N, et al. Disease- and cell-specific expression of GP73 in human liver disease. Am J Gastroenterol. 2004;99:1087-95.
- Kladney RD, Cui X, Bulla GA, et al. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology. 2002;35(6):1431-40.
- Kawamoto S, Moriwaki K, Nakagawa T, et al. Overexpression of α1, 6-fucosyltransferase in hepatoma enhances expression of Golgi phosphoprotein 2 in a fucosylation-independent manner. Int J Oncol. 2011;39(1):203-08.
- Hou SC, Xiao MB, Ni RZ, et al. Serum GP73 is complementary to AFP and GGT-II for the diagnosis of hepatocellular carcinoma. Oncol Lett. 2013;6(4):1152- 58.
- Giannelli G, Antonaci S. New frontiers in biomarkers for hepatocellular carcinoma. Dig Liver Dis 2006;38(11):854- 59.
- Riener MO, Stenner F, Liewen H, et al. Golgi phosphor- protein 2(GOLPH2) expression in liver tumors and its value as a serum marker in hepatocellular carcinomas. Hepatology. 2009;49(5):1602-09.
- Ozkan H, Erdal H, Tutkak H, et al. Diagnostic and prognostic validity of Golgi protein 73 in hepatocellular carcinoma. Digestion. 2011;83(1-2):83-88.
- Tian L, Wang Y, Xu D, et al. Serological AFP/Golgi protein 73 could be a new diagnostic parameter of hepatic diseases. Int J Cancer. 2011;129(8):1923-31.
- Sun Y, Yang H, Mao Y, et al. Increased Golgi protein 73 expression in hepatocellular carcinoma tissue correlates with tumor aggression but not survival. J Gastroenterol Hepatol. 2011;26(7):1207-12.
- Bao YX, Cao Q, Yang Y, et al. Expression and prognostic significance of golgi-glycoprotein 73(GP73) with epithelial- mesenchymal transition (EMT) related molecules in hepatocellular carcinoma(HCC). Diagn Pathol. 2013;8:197.
- Gu Y, Chen W, Zhao Y, et al. Quantitative analysis of elevated serum Golgi protein-73 expression in patients with liver diseases. Ann Clin Biochem. 2009;46(Pt 1):38-43.
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29-36.
- Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases:Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208-36.
- Oka H, Tamori A, Kuroki T, et al. Prospective study of alpha- fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatol. 1994;19(1):61-66.
- Pateron D, Ganne N, Trinchet JC, et al. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol. 1994;20:(1) 65-71.
- Zoli M, Magalotti D, Bianchi G, et al. Efficacy of a surveillance program for early detection of hepatocellular carcinoma. Cancer. 1996;78(5):977-85.
- Li X, Wu K, Fan D. Serum Golgi Phosphoprotein 2 level: a better marker than alpha- fetoprotein for diagnosing early hepatocellular carcinoma. Hepatology. 2009;50(1):325.
- Sangiovanni A, Romeo R, Prati GM, et al. Serum expression of lectin reactive α-fetoprotein, des-γ- carboxy prothrombin, Golgi protein-73 antigen and antibody, for the diagnosis of hepatocellular carcinoma. Hepatology. 2007;46(supp l1):406A.
- Lok AS, Sterling RK, Everhart JE, et al. HALT-C trial group. Des-gamma-carboxy prothrombin and alphafetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138(2):493-502.
- Yousuf F, Ding W, Richardson P, et al. PWE-135 does AFP predict survival in patients with hepatocellular carcinoma(hcc)? Gut. 2014;63(Suppl 1):A184.
- Zhang K, Song P, Gao J, et al. Perspectives on a combined test of multi serum biomarkers in China:towards screening for and diagnosing hepatocellular carcinoma at an earlier stage. Drug Discov Ther. 2014;8(3):102-109.
- Zhang Z, Zhang YR, Wang Y, et al. Alpha-fetoprotein-L3 and Golgi protein 73 may serve as candidate biomarkers for diagnosing alpha-fetoprotein-negative hepatocellular carcinoma. Onco Targets Ther. 2016;9:123-29.
- Fathy WM, Abo-Elela D, Hegazy O. Assessment the level of Golgi protein 73 and clusterin among Egyptian patients for detection of hepatocelluar carcinoma. J Am Sci. 2015;11(11):189-97.
- Marrero JA, Romano PR, Nikolaeva O, et al. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol.(2005);43(6):1007- 1012.
- Fimmel CJ, Wright L. Golgi protein 73 as a biomarker of hepatocellular cancer: Development of a quantitative serum assay and expression studies in hepatic and extra hepatic malignancies. Hepatology. 2009;49(5):1421-1423.
- Khalil FM, Negm SI, El-Assal MA, et al. Serum level of Golgi protein-73 as a diagnostic marker for hepatocellular carcinoma. Benha Med J. 2018;35(1):36-41.
- El-Shafie M, Fawzy A, Abd Al-Monem E, et al. Golgi protein 73 as a novel marker for early detection of HCC in Egyptian patients. Life Sci J. 2012;9(2):823-830.
- Wang NY, Wang C, Li W, et al. Prognostic value of serum AFP, AFP-L3, and GP73 in monitoring short-term treatment response and recurrence of hepatocellular carcinoma after radiofrequency ablation. Asian Pacific Journal of Cancer Prevention. 2014;15(4):1539-1544.
- Ai N, Liu W, Li Zg, et al. High expression of GP73 in primary hepatocellular carcinoma and its function in the assessment of transcatheter arterial chemoembolization. Oncology letters. 2017;14(4):3953-3958.
- Dong M, Chen ZH, Li X, et al. Serum golgi protein 73 is a prognostic rather than diagnostic marker in hepatocellular carcinoma. Oncology Letters. 2017;14(5):6277-6284.