Review Article - Journal of Cell Biology and Metabolism (2018) Histology and Cell Biology
Evaluation of chemerin and leptin in serum of chronic hepatitis C patients.
Phebe Lotfy Abdel-Messeih*, Heba Hosny Mansour, Dalia Ramzy Ibrahim
Egyptian Atomic Energy Authority Cairo, Egypt
- *Corresponding Author:
- Phebe L Abdel-Messeih
National Center for Radiation Research and
Technology
Atomic Energy Authority
Cairo
Egypt
Tel: +201225820326
E-mail: febylotfy@yahoo.com
Accepted date: February 20, 2018
Citation: Abdel-Messeih PL, Mansour HH, Ibrahim DR. Evaluation of chemerin and leptin in serum of chronic hepatitis C patients. J Histol Cell Biol. 2018;1(1):8-12
Abstract
Chronic hepatitis C (CHC) is considered a metabolic liver disease. Several non-invasive biomarkers that mirror liver injury progression have been proposed. Child pugh score including: Albumin, bilirubin and prothrombin time (INR) is used to assess prognosis in CHC. The aim of this study was to find out if the novel adipokines, leptin and chemerin, could be considered as additional tools for assessment of prognosis of CHC. The study was performed on 20 male patients with CHC, aged between 40 and 60 (mean value 47.5 ? 11.1). Exclusion criteria included: hepatitis B, human immunodeficiency virus (HIV) co-infection, hepatocellular carcinoma, diabetes mellitus, obesity and renal or heart failure. All patients and controls underwent the following laboratory investigations: Chemerin and leptin, alphafetoprotein, liver function tests, polymerase chain reaction (PCR), blood glucose level, fasting insulin and insulin resistance. A significantly higher serum chemerin and leptin concentrations were recorded in CHC patients compared to the control. The significant negative correlation recorded between chemerin and albumin, as well as between leptin and albumin demonstrates that a significant increase in the level of these adipokines occurs as liver function worsens. Moreover, a significant positive correlation between chemerin and prothrombin time (INR) indicates that cardiovascular risk was higher in CHC patients compared to controls. It could be concluded that chemerin and leptin may be considered of prognostic significance in CHC which might prevent the need to liver biopsy
Keywords
Chronic hepatitis c, Chemerin, Leptin.
Introduction
There are approximately 170-200 million people in the world infected with hepatitis C virus (HCV) and 350,000 deaths each year are caused by HCV infection [1]. HCV infection is associated with the development of insulin resistance and hepatic steatosis [2]. Through these metabolic pathways (as well as through other hypothesized mechanisms that involve lipid metabolism, systemic inflammatory signals, and endothelial dysfunction), chronic HCV infection also contributes to significant systemic cardiovascular morbidity and mortality [3]. It is also well recognized that the presence of metabolic disturbance in patients infected with HCV accelerates the development of liver disease [4], hepatocellular carcinoma and the progression to advanced fibrosis [3].
Adipose tissue acts as an active endocrine organ. The cytokines produced by adipose tissue (adipocytokines) have a distinct role in modulating inflammatory response and insulin sensitivity [5]. Some adipocytokines have been said to influence liver fibrosis progression [6]. Thus, adipokines profile, together with insulin resistance, seems to play a distinct role in the pathogenesis of liver disease [7].
Leptin is a 16-kDA protein encoded by the obese (ob) gene. It is produced primarily in the adipocytes of white adipose tissue. One of the major actions of leptin is the control of energy balance by binding to receptors in the hypothalamus, leading to reduction in food intake and elevation in temperature and energy expenditure. In addition, increasing evidence suggests that leptin, may play an important role in cardiovascular and renal regulation [8]. Leptin receptors (ObR) are expressed in a broad range of peripheral tissues, including the liver, and have isoforms as a result of alternative splicing. The level of secretion of leptin is associated with the fat mass and provides anti-obesity signals, regulating food intake, sympathetic tone and energy expenditure in conditions of energy excess. Leptin promotes fibrogenesis both by direct and indirect mechanisms. The direct effect is by activation of hepatic stellate cells (HSCs) [9,10]. However, the indirect mechanism is by activation of Kupffer cells, macrophages and sinusoidal endothelial cells through upregulation of transforming growth factor beta-1(TGFβ1) production. Leptin increases the expression of procollagen I, TGFβ1 and α-smooth muscle actin in the activated HSCs [11]. Moreover, leptin promotes fibrogenesis by stimulation of tissue inhibitors of metalloproteinases (TIMPs) and inhibition of matrix metalloproteinase-1(MMP-1) [12].
The family of adipokines is still growing and novel adipokines such as chemerin, visfatin, and vaspin were recently described in association with different diseases [13-15].
Chemerin is a new adipokine, which expression has been found in a number of tissues including those of the liver, pancreas and lungs, as well as in adipose tissue. The liver is one of the main chemerin-secreting organs [16], although adipose tissue produces more. It has been shown to be associated with body mass index (BMI), plasma triglycerides (TG), blood pressure and insulin resistance [17]. Chemerin is a chemoattractant protein and it has a role in adipogenesis, angiogenesis and glucose homeostasis. Chemerin receptors are expressed in the liver suggesting that chemerin may be relevant in liver physiology and pathophysiology [18]. It reveals chimeric nature; being both pro-inflammatory and anti-inflammatory. The pro-inflammatory action is exerted by activating and recruiting natural killers (NK) cells and macrophages into inflamed tissue [19], however the anti-inflammatory action occurs by inhibiting synthesis of pro-inflammatory mediators and stimulating adiponectin expression [20]. Kukla et al. confirmed a marked expression of chemerin in the liver of CHC patients [21]. In the same line Imai et al. recorded that platelet counts and total bilirubinlevels are associated with serum chemerin levels in patients with hepatocellular carcinoma [22].
The frequency of hepatic infection in males was reported to be higher than females with a male to female ratio of 3:8:1 [23]. Furthermore, the rates of fibrosis progression in chronic hepatitis C are significantly higher in males than females [24,25], probably due to the anti-fibrogenic effect of oestrogen via inhibition of stellate cells [24], and the higher hepatitis C virus clearance rate in women than men [26].
In view of the previous considerations, male patients were selected to investigate if the novel adipokines, leptin and chemerin, could be considered additional tools for assessment of prognosis of CHC in male patients.
Materials and Methods
The present study was performed on 20 male patients with Chronic hepatitis C viral infection (CHC), from those attending The Tropical Medicine Department- Cairo University and categorized as CHC patients with persistently elevated alanine aminotransferase (ALT) activity for at least 6 months, aged between 40 and 60 with a mean value of 47.5 ± 11.1, and BMI mean value26.94 ± 0.94 kg/m2. Exclusion criteria included: hepatitis B or HIV co-infection, hepatocellular carcinoma, diabetes mellitus, obesity and renal or heart failure.
Fasting blood samples were collected from all subjects in blank vacutainers (left to clot, centrifuged and sera separated and stored at -20 till assay date). Moreover, 2ml were collected for assay of prothrombin time on aqueous trisodium citrate dehydrate to obtain plasma samples for testing INR within 2 hours as the preferred schedule. The diagnosis of CHC was confirmed by the presence of serum HCV-RNA assayed with the reverse transcription polymerase chain reaction (RT-PCR) method. All patients were native for the antiviral treatment. The control group consisted of 20 healthy male volunteers without anti-HCV antibodies, hepatitis B surface (HBs) antigen negative and HIV negative, with normal ALT, age mean value 45.9 ± 11.8 years and BMI mean value 25.53 ± 2.21 kg/m2). All the healthy volunteers had normal fasting glucose levels.
All procedures performed were according to the ethical standards of the institutional and national research committee given in the Declaration of Helsinki1964, as revised in 2013. The authors declare no conflicts of interest. Informed consent was obtained from all participants in the study.
Biochemical analysis
The levels of chemerin and leptin in serum were assessed in duplicate by competitive immunoenzymatic method with the commercially available ELISA kits. Human Chemerin Quantikine ELISA Kit, supplied by R&D Systems, Inc [27], and Human Leptin ELISA Kit supplied by Diagnostic Biochem. Canada Inc [28]. Insulin concentration was measured by Siemens Healthcare Diagnostics Insulin EIA Kit, ADVIA Centaur and ReadyPack Bayswater Victorie 5153, Australia [29]. The biochemical parameters aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, albumin and fasting blood sugar were measured using routine methods on Hitachi 971 instrument (Roche Diagnostics GmbH, D, 68298 Mannheim). The degree of insulin resistance (IR) was calculated according to the homeostasis model assessment for IR (HOMAIR) by the formula: fasting insulin level (mIU/L) multiplied by fasting glucose level (mg/dL) divided by 405. The non invasive marker of liver tissue alterations, aspartate aminotransferase/ alanine aminotransferase ratio (AAR)=AST/ALTwas calculated. Prothrombin time was determined by the Hospitex single channel coagulometer (Hospitex via S. Piero a Quaracchi, 224-50145 FIRENZE-ITALY). Serum alphafetoprotein was assayed to exclude hepatocellular carcinoma by immuneradiometric assay (IRMA) using Coat-A-Count AFP IRMA kit provided by Diagnostic products cooperation(DPC), 157700 west 96 m street Los Angeles, CA90045-5597) [30]. Hepatitis markers were detected by Microparticle Enzyme Immunoassay (MEIA) using the AxSym auto analyzer provided by Abbott Laboratories Diagnostic Division Max-Planck-Ring 65205 Germany [28]. These were done to exclude co-existent infection with other viruses in patients group and to ensure that the controls are sero-negative for HBV, HCV and HIV. Serum HCV-RNA was assayed with the RT-PCR method using Amplicor Roche/Promega v.2 Diagnostic Test, Branchburg, NJ, USA, and viral load by signal amplification nucleic acid probe assay for the quantitation of human hepatitis C viral RNA using Bayer Versant HCV RNA 3.0 Assay (bDNA) provided by Bayer Diagnostics, Berkeley, CA, USA in CHC patients.
Statistics
The results were expressed as mean ± standard deviation. The statistical difference between groups was analyzed using Student's t-test. Pearson's correlation analysis was performed to determine the relationships between variables. The results were considered significant at p<0.05. The Statistical Package for the Social Sciences, Version 15 software was used, and the presentations performed using Microsoft Excel 2007.
Results
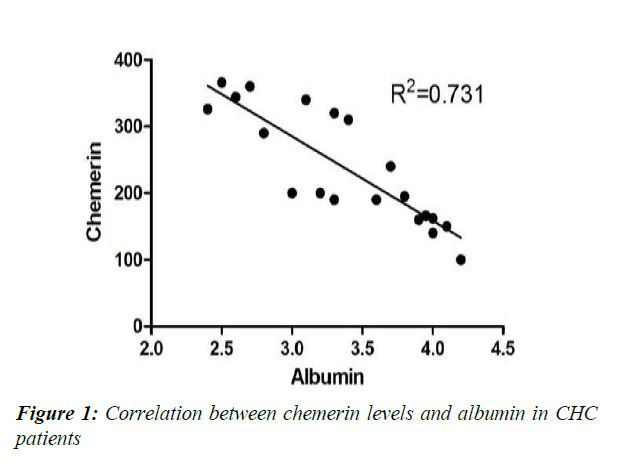
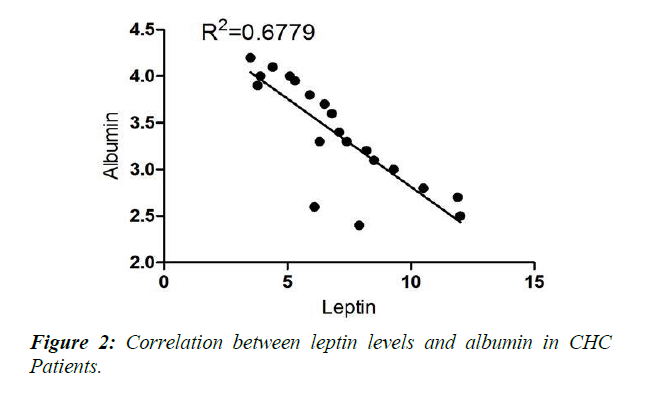
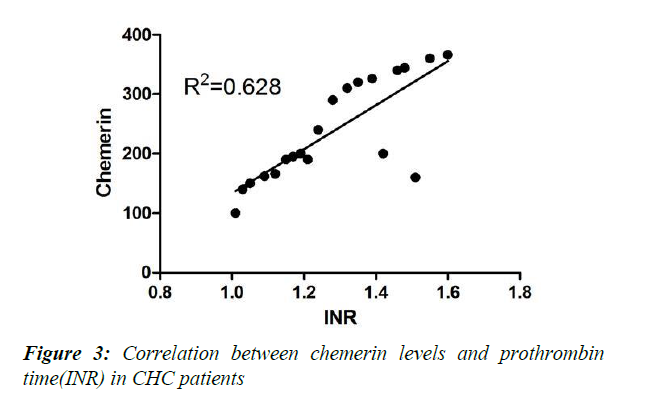
Physical characteristics, glycaemic state, adipocytokine levels and laboratory investigations of the studied group of chronic hepatitis C patients (CHC) compared to controls are illustrated in Tables 1-3. No significant difference was detected between controls and CHC patients regarding blood sugar and fasting insulin levels. BMI was higher in CHC patient yet still non obese (Table 1). Both measured adipocytokines-leptin and chemerin-were significantly higher in CHC patients compared to controls (Table 2). Serum albumin level was significantly lower in CHC patients than controls (p<0.001). All other measured liver functions were significantly higher in CHC patients than controls (p<0.001) except the increase of alkaline phosphatase that was insignificant (p>0.05) (Table 3). The correlation analysis statistics revealed significant negative correlation between chemerin and albumin (r2=0.731) as shown in Figure 1. Also, significant negative correlation was found between leptin and albumin (r2=0.677) as shown in Figure 2. However, significant positive correlation between chemerin and INR (r2=0.628) was found as shown in Figure 3.
Table 1. Physical characteristics and glycaemic state of CHC patients compared to controls.
| Parameters | Control | CHC | P Value |
|---|---|---|---|
| Fasting insulin (IU/ml) | 9.5 ± 3.358 | 11.35 ± 1.124 | >0.05 |
| Fasting blood glucose (mg/dl) | 88.55 ± 6.992 | 89.5 ± 11.91 | >0.05 |
| BMI (non-obese) (kg/m2) | 25.53 ± 2.215 | 26.94 ± 0.9422** | <0.005 |
| HOMA-IR | 2.132 ± 0.892 | 2.516 ± 1.107 | >0.05 |
**Highly statistically significant.
p>0.05=non significant.
Table 2. Adipocytokine levels in CHC patients compared to controls.
| Parameters | Control | CHC | P Value |
|---|---|---|---|
| Leptin (ng/ml) | 3.720 ± 0.5782 | 7.020 ± 2.507*** | <0.0001 |
| Chemerin (ng/ml) | 87.8 ± 22.11 | 237.5 ± 85.24*** | <0.0001 |
***Highly statistically significant.
Table 3. Laboratory assessment of liver functions and related parameters in CHCpatients compared to controls.
| Parameters | Control | CHC | P Value |
|---|---|---|---|
| Alpha-fetoprotein (ng/ml) | 3.890 ± 2.649 | 158.7 ± 93.31*** | <0.0001 |
| Alkaline phosphatase (IU/L) | 88.45 ± 51.04 | 100.5 ± 47.65 | >0.05 |
| Non invasive AST/ALT | 1.239 ± 0.4555 | 0.8555 ± 0.2724** | <0.05 |
| Albumin (gm/dl) | 4.27 ± .5192 | 3.378 ± 1.5755*** | <0.005 |
| ALT (IU/L) | 16.73 ± 3.816 | 75.05 ± 41.29*** | <0.0001 |
| AST (IU/L) | 20.20 ± 6.940 | 63.4 ± 49.23** | <0.005 |
| Total bilirubin (mg/dl) | 0.7480 ± 0.239 | 1.105 ± 0.319*** | <0.001 |
| INR | 1.053 ± 0.064 | 1.281 ± 0.182*** | <0.0001 |
| Quantitative PCR (IU/L) | 1715 ± 973.3 |
* *, ***Highly statistically significant.
p>0.05=non-significant.
Discussion
CHC viral infection causes liver inflammation by complex molecular pathways including direct viral effects and indirect mechanisms involving cytokine pathways and oxidative stress induction [31]. Multiple studies reported the contribution of different adipocytokines to the development of metabolic abnormality in CHC and their role in fibrogenesis yet some results seem to be contradictory and confusing [32].
In the current study, the goal was to concentrate on CHC as a virally derived metabolic abnormality. Therefore, the study was performed on only normal weight and overweight but not obese, and on normoglycaemic CHC patients, to avoid the possible influence of obesity or diabetes as metabolic abnormalities with direct effect on adipokine profile. Several studies tried to explain the reasons of high prevalence of diabetes mellitus in chronic hepatitis C patients. Explanations proposed included HCV induced steatosis, hepatic insulin resistance or immune mediated extrahepatic diabetogenic effect [33]. High serum levels of chemerin were reported in humans with both type one and type two diabetes, or impaired glucose tolerance [34].
Hepatocellular carcinoma (HCC) is one of the most frequently occurring cancers worldwide. The conventional risk factors include persistent infection with hepatitis viruses and alcohol consumption [35]. Chemerin was reported to play an important role in the development and progression of HCC [36]. Thus, to avoid the interference of chemerin alterations, AFP which is still the best available screening serological test for HCC [37], was performed to exclude co-exsistent HCC. The results obtained showed that the level of AFP in CHC patients, although higher than in the control group it was still lower than 200 ng/ml the best specificity level for the determination of HCC in CHC patients [38].
Liver biopsy represents the gold standard for evaluating liver damage, but identification of noninvasive markers that mirror liver injury progression is the actual goal. Several noninvasive markers have been proposed. Child pugh score is used to assess prognosis in chronic liver disease. Parameters for the determination of child pugh score include: Albumin, bilirubin and prothrombin time (INR). Other noninvasive markers include AAR(AST/ALT ratio) [39]. This study tried to measure novel adipokines (chemerin and leptin) to be used as non-invasive markers and studied the presence of correlations between the markers of liver prognosis and the novel adipokines.
In the present study, significantly higher serum leptin concentrations were found in CHC patients compared to the control. These results are in agreement with the previous findings of Sell et al. and Tiftikci et al. [40,41], and could be attributed to the role of leptin in promoting fibro genesis [10]. Significantly increased chemerin levels were recorded also in CHC patients, which is in line with the findings obtained by Kukla et al. However, chemerin levels were negatively correlated with necro-inflammatory grade [42]. The results could be interpreted by the fact that chemerin is a chimeric protein synthesized mainly in hepatocytes and fat tissue with both pro-inflammatory and anti-inflammatory effects. It acts as a chemoattractant for macrophages, dendritic cells (DC), and NK cells and stimulates macrophage adhesion to vascular cell adhesion molecule 1 (VCAM1) and fibronectin [43]. It was claimed as an important element in the pathogenesis of CHC especially in the inflammatory mechanism although further investigations are required to clarify its role [32].
The results of the present study revealed a significant negative correlation between chemerin and albumin (r2=0.731) as shown in Figure 1, and a significant negative correlation leptin and albumin (r2=0-677) as shown in Figure 2. These findings demonstrated that significant increase in the level of these adipokines occurs as liver functions worsen as confirmed by the significant decrease of albumin and increase of INR.
Recent accumulating evidence suggests that CHC infection can increase cardiovascular risk. These data are strengthened by evidence identifying potential mechanisms linking CHC infection to vascular damage [44]. Several recent studies evaluated the frequency of cardiovascular involvement in CHC and documented that cardiovascular complications could be considered among the large scope of extrahepatic manifestations of CHC [45]. However, the presence of contrasting results not identifying any association between CHC infection and cardiovascular dysfunction provides uncertainty about a direct association of CHC infection with cardiovascular risk [44].
Chemerin is known to be responsible for coronary artery disease development together with many other factors because it is highly associated with inflammation and adipogenesis. The results of the present study showed a significant positive correlation between chemerin and prothrombin time (INR) (r2=0=628) as shown in Figure 3 indicating that cardiovascular risk is higher in CHC patients compared to controls.
Conclusion
The results obtained in the current study demonstrate that the serum chemerin and leptin levels increased in parallel with the worsening liver functional reserves in CHC patients and chemerin may help in the detection of increased cardiovascular risk in CHC patients. It could be concluded that chemerin and leptin may be considered as additional tools for assessment of prognosis of CHC and monitoring the virally derived metabolic abnormality.
References
- Marinho RT, Vitor S, Velosa J. Benefits of Curing Hepatitis C Infection. J Gastrointestin Liver Dis. 2014;23(1):85-90.
- Kralj D, Jukić LV, Stojsavljević S, et al. Hepatitis C Virus, Insulin Resistance, and Steatosis. J Clintranslhepatol. 2016;4(1):66-75.
- Wong RJ, Gish RG. Metabolic Manifestations and Complications Associated with Chronic Hepatitis C Virus Infection. Gastroenterolhepatol (NY) 2016;12(5):293-9.
- Lim TR. Metabolic Syndrome in Chronic Hepatitis C Infection: Does it Still Matter in the Era of Directly Acting Antiviral Therapy? Hepat Med. 2014;6:113-8.
- Rabe K, Lehrke M, Parhofer KG. Adipokines and Insulin Resistance. Molmed. 2008;12:741-51.
- Saxena NK and Anania FA. Adipocytokines and Hepatic Fibrosis. Trends Endocrinolmetab. 2015;26(3):153-61.
- Bertolani C, Marra F. The Role of Adipokines in Liver Fibrosis. Pathophysiology. 2008;15:91-101.
- Kshatriya S, Liu K, Salah A, et al. Review Article Obesity Hypertension: The Regulatory Role of Leptin. Int J Hypertens. 2011.
- Saxena NK, Ikeda K, Rockey DC, et al. Leptin in Hepatic Fibrosis: Evidence For Increased Collagen Production in Stellate Cells and Lean Littermates of Ob/Ob Mice. Hepatology 2002;35(4):762-71.
- Marra F. Leptin and Liver Tissue Repair: Rodent Models Provide the Answers? J Hepatol. 2007;46:12-8.
- Bertolani C, Marra F. Role of Adipocytokines in Hepatic Fibrosis. Curr Pharm Des. 2010;16:1929-40.
- Cao Q, Mak KM, Lieber CS. Leptin Repress Matrix Metalloproteinase-1 Gene Expression in Lx2 Human Hepatic Stellate Cells. J Hepatol. 2007;46:124-33.
- Bekaert M, Verhelst X, Geerts A, et al. Association Of Recently Described Adipokines with Liver Histology in Biopsy-Proven Non-Alcoholic Fatty Liver Disease: A Systematic Review, Obesity Review 2016;17(1):68-80.
- Azamar-Llamas D, Hernández-Molina G, Ramos-Ávalos B, et al. Adipokine Contribution to the Pathogenesis of Osteoarthritis. Mediators Inflamm. 2017.
- Adolph TE, Grander C, Grabherr F, et al. Adipokines and Non-Alcoholic Fatty Liver Disease, Multiple Interactions. Int J Mol Sci. 2017;18.
- Krautbauer S, Wanninger J, Eisinger K, et al. Chemerin is Highly Expressed in Hepatocytes and is Induced in Non-Alcoholic Steatohepatitis Liver. Exp Mol Pathol. 2013;95:199-205.
- Bozaoglu K, Bolton K, McMillan J, et al. Chemerin is a Novel Adipokine Associated with Obesity and Metabolic Syndrome. Endocrinology. 2007;148(10):4687-94.
- Buechler C. Chemerin in Liver Diseases. Endocrinol Metab Synd. 2014;3:4.
- Moretta A, Marcenaro E, Parolini S, et al. Nk Cells at the Interface between Innate and Adaptive Immunity. Cell Death Differ. 2008;15(2): 226-33.
- Yoshimura T, Oppenheim JJ. Chemerin Reveals a Chimeric Nature. J Exp Med. 2008;205(10):2187-90.
- Kukla M, Adamek B, Waluga M, et al. Hepatic Chemerin and Chemokine-Like Receptor 1 expression in Patients with Chronic Hepatitis C. Biomed Res Int. 2014.
- Imai K, TakaiK, Hanai T, et al. Impact of Serum Chemerin Levels On Liver Functional Reservesand Platelet Counts in Patients with Hepatocellular Carcinoma. Int J Mol Sci. 2014;15:11294-306.
- Baig S. Gender Disparity in Infections of Hepatitis B Virus. J Coll Physicians Surg Pak. 2009;19(9):598-600.
- Codes L, Asselah T, Cazals-Hatem D, et al. Liver Fibrosis in Women with Chronic Hepatitis C: Evidence for the Negative Role of the Menopause and Steatosis and the Potential Benefit of Hormone Replacement Therapy. Gut. 2007;56(3):390-95.
- Collazos J, Carton JA, Asensi V (2011) Gender Differences in Liver Fibrosis and Hepatitis C Virus-Related Parameters in Patients Co-Infected with Human Immunodeficiency Virus. Curr HIV Res. 2007;9(5):339-45.
- Bakr I, Rekacewicz C, El Hosseiny M, et al. Higher Clearance of Hepatitis C Virus Infection in Females Compared with Males. Gut 2006;55(8):1183-87.
- Monnier J, Lewén S, O'Hara E, et al. Expression, Regulation, and Function of Atypical Chemerin Receptor Ccrl2 on Endothelial Cells. J Immunology 2012;189(2):956-67.
- Engvall E and Perlmann P. Enzyme Linked Immunosorbent Assay (Elisa): Quantitative Assay of Immunoglobulin G Immunohistochemistry 1971;8:871-4.
- Zhao W and Alkon DL. Role of Insulin and Insulin Receptor in Learning and Memory. Mol Cell Endocrinology. 2001;177(1,2):125-34.
- Dudley RA, Edwards P. Guidelines for Immunoassay Data Processing. Clinical Chemistry. 1985;31:1264-71.
- Lindenbach BD and Rice CM Unravelling Hepatitis C Virus Replication from Genome to Function. Nature. 2005;436:933-8.
- Andrea RM, Cristina P, Victoria A, et al. The Role of New Adipokines in the Pathogenic Mechanisms of Chronic Hepatitis C. Therapeutic Pharmacy and Clinical Toxicology. 2012;3:151-4.
- Hickman IJ, Clouston AD, Macdonald GA. Effect of Weight Reduction on Liver Histology and Biochemistry in Patients with Chronic Hepatitis C. Gut 2002;51:89-94.
- Stuart AAV, Schipper HS, Tasdelen. Altered Plasma Adipokine Levels and in Vitro Adipocyte Differentiation in Pediatric Type 1 Diabetes. J Clin Endocrinol Metabolism. 2012;97(2):463-72.
- Shimizu M, Tanaka T and Moriwaki H. Obesity And Hepatocellular Carcinoma:Targeting Obesity-Related Inflammation for Chemoprevention of Liver Carcinogenesis. Immunopathology 2013;35,191-202.
- Lin W, Chen YL, Jiang L, et al. Reduced Expression of Chemerin is Associated with a Poor Prognosis and A Lowed Infiltration of Both Dendritic Cells and Natural Killer Cells. Clin Lab. 2011;57(11,12):879-85.
- Sarwar S, Khan AA, Tarique S. Validity of Alpha Fetoprotein for Diagnosis of Hepatocellular Carcinoma in Cirrhosis. Journal of the College of Physicians and Surgeons Pakistan. 2014;24(1):18-22.
- Arrieta O, CachoB, Morales-Espinosa D. The Progressive Elevation of Afp for the Diagnosis of Hepatocellular Carcinoma in Patients with Liver Cirrhosis. BMC Cancer. 2007;7(28):1471-2407.
- Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, Management, and Treatment of Hepatitis C: An Update. Hepatology. 2009;49:1335-74.
- Sell, H, Divoux A, Poitou C, et al. Chemerin Correlates with Markers for Fatty Liver in Morbidly Obese Patients And Strongly Decreases After Weight Loss Induced by Bariatric Surgery. J Clin Endocrinol Metab. 2010;95:2892-96.
- Tiftikci A, Atug O, Yilmaz Y, et al. Serum Levels of Adipokines in Patients with Chronic Hcv Infection: Relationship with Steatosis and Fibrosis. Arch Med Res. 2009;40(4):294-302.
- Kukla M, Mazur W, Bułdak RJ. Potential Role of Leptin, Adiponectin and Three Novel Adipokines-Visfatin, Chemerin and Vaspin-In Chronic Hepatitis. Molecular Medicine. 2011;17(11):1397-1410.
- Hart R, Greaves DR. Chemerin Contributes To Inflammation by Promoting Macrophage Adhesion To Vcam-1 and Fibronectin Through Clustering of Vla-4 and Vla-5. J Immunol. 2010;185:3728-39.
- Petta S. Hepatitis C Virus and Cardiovascular: A Review. J Advanced Research. 2017;8(2):161-8.
- Domont F and Cacoub P. Chronic Hepatitis C Virus, A New Cardiovascular Risk Factor. Liver Int. 2016;36(5):621-7.