Research Article - Biomedical Research (2017) Volume 28, Issue 4
Evaluation of black cumin seeds hexane extract as reactive oxygen intermediates (ROI) and phagocytic activity modulator in DMBA inducedrats
Titiek Hidayati1#*, Ardi Pramono2#, Ikhlas Muhammad Jenie3# and Marsetyawan Hne Soesatyo4#1Department of Epidemiology, Public Health and Family Medicine, Faculty of Medicine and Health Science, Universitas Muhammadiyah Yogyakarta, Yogyakarta, Indonesia
2Department of Biochemistry and Anaesthesia, Faculty of Medicine and Health Science, Universitas Muhammadiyah Yogyakarta, Yogyakarta, Indonesia
3Department of Physiology, Faculty of Medicine and Health Science, Universitas Muhammadiyah Yogyakarta, Yogyakarta, Indonesia
4Department of Histology, Faculty of Medicine, Gadjah Mada University, Yogyakarta, Indonesia
#These authors contributed equally to this work
- *Corresponding Author:
- Titiek Hidayati
Department of Epidemiology, Public Health and Family Medicine
Faculty of Medicine and Health Science
Universitas Muhammadiyah Yogyakarta, Indonesia
Accepted date: September 17, 2016
Abstract
Dimethylbenzanthracene (DMBA) compounds were proven to suppress bone marrow activity, immunosuppressive, and genotoxic and carcinogenic. Black cumin seed extract was used as an immunomodulator, which can be developed as a great therapeutic agent for adjuvant chemotherapy in cancer patients as an immunomodulatory agent. The aim of this study is to determine the effect of black cumin seed hexane extract (BCSHE) as an immunomodulator in SD rat’s peritoneal macrophages induced by DMBA or without DMBA. In vitro experimental study was performed on rat’s peritoneal macrophages cultures induced by DMBA and without DMBA. BCSHE were administered to cultures in five different doses; 1, 5, 25, 125 and 625 μg/ml with control media, tween, and positive control thymoquinone. The test substance was added after the macrophages were activated and then measured by using latex phagocytosis method while ROI secretion assay was measured by using NBT assay method. Results showed that macrophage activation by BCSHE solution increased ROI secretory activity in both SD rat’s macrophage induced by DMBA and without DMBA. BCSHE could increase phagocytic activity in-vitro. It can be concluded that BCSHE can increase ROI secretion and phagocytic activity on SD rat’s peritoneal macrophages induced by DMBA and without DMBA.
Keywords
Black cumin seeds hexane extract, Dimethylbenzanthracene, Immunomodulator, Macrophages
Introduction
Thymoquinone is the primary active substance of black cumin (Nigella sativa) seeds which is used as traditional medicine to repair the immune system, enhance stamina, and as an antiinflammatory. Black cumin seed extract inhibited the cyclooxygenase pathway and a 5-lipooxygenase in arachidonic metabolism on leukocytes of rat’s peritoneal cavity [1]. Furthermore, Akrom et al. proved that black cumin seeds extract administration to Sprague-Dawley (SD) rats before and during dimethylbenzanthracene (DMBA) induction prevented mammary carcinogenesis, increased the number of leukocytes, and the viability of experimental animals [2]. ROI is one of the pleiotropic compounds. Macrophages are activated by pathogens secreted by ROI to kill bacteria. However, the excessive level of ROI become a reactive radical and disrupt the normal cells. DMBA compounds are known as carcinogens, immunosuppressive, and a source of reactive radicals. Other study reported that DMBA induction inhibited inducible nitric oxide synthase (iNOS) expression, NO, and interleukin 12 (IL-12) secretion by macrophages [3]. NFκB and CCAAT/enhancer binding protein (C/EBP) depletion alongside with a decrease in function and activity of rat’s peritoneal macrophages induced by DMBA [3]. DMBA exposure on in vivo and in-vitro proven to reduce the activity of macrophages [4].
Administration of thymoquinone to the in-vitro macrophage cell cultures activated Toll-Like Receptor 4 (TLR-4) through the activation of the sialidase NEU-4 enzyme. Administration of thymoquinone in rats increase levels of IL-1, TNFα, and IL-6, even without induced LPS, it proved that thymoquinone increased phagocytic activity of macrophages and proinflammatory in macrophage cells [5]. Thymoquinone and unsaturated fatty acid compound in black cumin seed extract are assumed to have anti-oxidative and immunostimulatory activity which expected to activate antioxidant enzymes of rat’s macrophages exposed by DMBA and impede the immunosuppressive properties [6]. Although black cumin seed is a potential immunomodulatory agent, including for people with cancer, currently there is no evidence how it influences ROI secretion activity of SD rat’s in vitro DMBA induced macrophages. This study is to determine the effect of black cumin seed hexane extract (BCSHE) on ROI secretion activity of SD rats in vitro DMBA induced macrophages.
Materials and Methods
Identification, extraction and standardization of black cumin seed with thymoquinone
Black cumin seeds obtained from a certified traditional medicine sellers in Semarang were identified at the Pharmacy and Biology Laboratory, Gajah Mada University. Extraction was done through maceration method. Different level of solvent was applied as maceration strategy. 500 g of black cumin seeds powder was processed maceration with 1 L hexane solvent for 24 hours. Thus, it was stirred for 30 minutes and incubated overnight. The extract then was filtered using Buchner funnel to get the filtrate. This maceration process was done with three replication. Three filtrates then were mixed and evaporated in a rotary evaporator at 50°C up to get gel extract. Gel extract then was evaporated in disk plate using water bath. Thus, the crude extract was collected in the bottle [2]. Standardization of black cumin seed hexane extract (BCSHE) with thymoquinone was done by KLT-densitometry method to get the specific grade of thymoquinone in black cumin seed extract [7]. The assay of unsaturated and saturated fatty acid of BCSHE was done by Gas Chromatography/Mass Spectroscopy (GCMS). Measured parameters on a standardized black cumin seed hexane extract were the percentage of thymoquinone and unsaturated fatty acids. Standardization procedure of N. sativa as the extract is as described by earlier researchers [7].
Effectiveness test of black cumin seed hexane extracts (BCSHE) as immunomodulator
Female SD rats aged 3-4 weeks old, weighed around 100-140 g were obtained from the Biological Sciences Laboratory, Gajah Mada University. Experimental animals were kept in 50 × 30 × 20 cm metal cages individually, fed with pellet 529, and water moderately [2]. Parameters measured on immunomodulatory and anti-oxidative activity test was the ROI secretory activity of macrophages on healthy SD rats and DMBA-induced SD rats. ROI secretory activity of macrophages is determined by NBT assay [2]. All activities and treatments on experimental animals were performed in accordance with procedures approved by the ethics committee on Animal Testing Laboratory Gajah Mada University. Peritoneal macrophage was taken from 11/12 months SD rats.
DMBA induction and macrophage isolation: Oral administration of DMBA (1 × 15 mg) was given to SD rats [7]. After three days, 5 rats from each group was dissected by using narcosis chloroform. Rats were placed in supine position, the abdomen was opened and peritoneum sheath was cleaned by using 70% ethanol, then 10 ml of cold RPMI medium was injected into the peritoneal cavity, waited for 3 minutes and shook it slowly. Peritoneal fluid was removed from the peritoneal cavity by pressing the cavity with two fingers, the fluid was aspirated with a syringe injection, the lean and nonfatty part was selected. The aspirate was centrifuged at 1200 rpm, 4°C, for 10 min. The supernatant was discarded and the bottom sediment was taken, and then the pellet was resuspended in RPMI-1640 (Gibco) (contained 10% FBS, 1 mM sodium Bicarbonate, 2 mM L-Glutamine, 100 μl Penicillin and 0.5 mg streptomycin, 95% ethanol, heparin, sodium oxalate, and distilled water). The number of cells was calculated using a hemocytometer and the cell viability was determined by trypan blue solution, and then added to a complete medium to obtain a cell suspension with a 2.5 × 106 cell/ml. Microculture suspension cells were grown in 24 wells and given round coverslips. Each of the wells was filled by 200 microliters (5 × 105 cells) and incubated in a 5% CO2 incubator, 37°C, for 30 minutes. After that, it was added by 1 ml complete medium and incubated in a 5% CO2 incubator, 37°C for 2 hours. Cells were washed twice with 1 ml RPMI for each well and incubated in complete medium for 24 hours.
Black cumin seed hexane extract (BCSHE) to macrophage cultures: Black cumin seed hexane extract (BCSHE) was dissolved in DMSO, vortex and sonicated for homogenisation before given to macrophage culture. Black cumin seed extract with five different concentrations (1, 5, 25, 125 and 625 μg/ml) were added in macrophage cultures, 5 μg/ml thymoquinone was added in positive control group macrophage cultures while the media control group got an additional growth media. It was incubated for 2 hours in a 5% CO2 incubator, at 37°C. After that, the cells were washed twice with 1 ml RPMI each well and then phagocytic activity and the secretion of ROI examinations was done [2]. ROI secretion activity test using NBT assay: ROI secretion by peritoneal macrophage was measured by NBT reduction assay [8]. To induce superoxide anion secretion, macrophage cell cultures were stimulated with Phorbol Mystrate Acetate (PMA) 125 ng/ml final concentration.
Macrophages that was cultured for 24 hours were washed twice with RPMI and 500 μl 1 mg/ml NBT and 125 ng/ml PMA in PBS, incubated in 5% CO2 incubator, 37°C, for 60 minutes. Subsequently, cells were washed with PBS three times, dried at room temperature, and fixated with absolute methanol for 30 seconds, and then dyed with 2% neutral red solution. The percentage of macrophage cells that showed NBT reduction was calculated using light microscopy with magnification 400X [2]. Phagocytic activity test using latex assay: Phagocytic analysis was done using by direct fixation procedure in microplates. Macrophages that was incubated in 5% CO2 incubator, 37°C, for 60 minutes. The cells were washed with PBS three times, dried at room temperature, and fixated with absolute methanol for 30 seconds. Then dyed with 20% Giemsa. The observation was done under the microscope with 400X magnification. Macrophage cells indicated with blue color. Percentage of phagocytic activity was measured minimum 100 observed cells. Phagocytic activity indicated by macrophage that phagocyte latex particles [7].
Data analysis
Data of ROI secretion was presented descriptively by stating the number, percentage, and average of each descriptive parameter but not followed by statistical analysis because the measurement was only performed twice.
Results
Determination, extraction, and standardization of black cumin seed
The result of maceration with hexane followed by distillation was clear yellow extract with fairly thin viscosity and distinctive volatile odor while the distillation method resulted in the yellowish brown extract with fairly thin viscosity and a distinctive volatile odor. Results of standardization of black cumin seed ethanol extract are presented in Table 1. Most of black cumin seed extract contents is vaporized extract (essential extract) and un-vaporized extract (fatty extracts).
| Content of black cumin seed | N | Crude extract market | Black Cumin Seed Hexane Extract |
|---|---|---|---|
| Thymoquinone | 3 | 2.72 ± 0.02 | 2.72 ± 0.03 |
| Fatty acid | 3 | 69.33 ± 1.16 | 78.00 ± 3.00 |
| Caproic acid | 3 | 0.21 ± 0.01 | 0.01 ± 0.00 |
| Capric acid | 3 | 0.06 ± 0.08 | 0.05 ± 0.00 |
| Lauric acid | 3 | 0.04 ± 0.05 | 0.02 ± 0.00 |
| Myristic acid | 3 | 0.18 ± 0.01 | 0.20 ± 0.00 |
| Palmitate | 3 | 12.28 ± 0.01 | 12.86 ± 0.08 |
| Palmitoleic | 3 | 0.29 ± 0.01 | 0.23 ± 0.01 |
| Stearate | 3 | 79.98 ± 0.02 | 11.30 ± 0.44 |
| Oleic | 3 | 0.07 ± 0.00 | 69.67 ± 0.68 |
| Linoleic | 3 | 2.86 ± 0.02 | 3.44 ± 0.09 |
| Linolenic | 3 | 0.11 ± 0.02 | 2.75 ± 0.10 |
Table 1. The content of black cumin seed extract set with TLC for thymoquinone and GCMS for unsaturated fatty extract.
More than 60% was fatty extract and less than 5% essential extract. Fatty extract of black cumin seed extract consisted of unsaturated fat and saturated fat. Most of the fatty acid was fatty extract macerated with hexane. Meanwhile, there were 74% distilled unsaturated fatty acid and 24% saturated fatty acid. The unsaturated fatty acids in the essential extract were: palmitoleic, oleic, linoleic and linolenic. Test results showed that maceration method with hexane, followed by steam distillation produced more favorable extract than the other methods.
Activity test of black cumin seed hexane extract against ROI secretory activity in macrophages
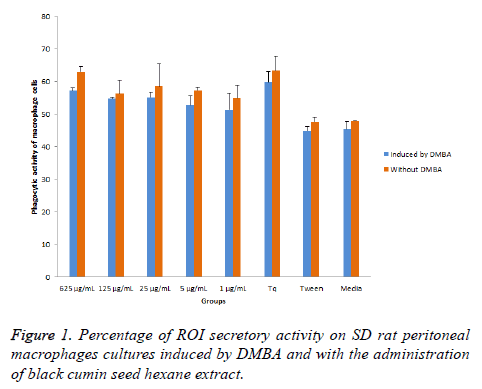
ROI secretion activity of SD rat macrophages induced by DMBA is presented in Figure 1, including the ROI secretory activity on SD rat peritoneal macrophages culture induced by DMBA or without DMBA with the administration of black cumin seed hexane extract. ROI secretory activity of SD rat peritoneal macrophages without DMBA was higher than those of induced by DMBA. Results showed that black cumin seed hexane extract (BCSHE) increased ROI secretion activity in peritoneal macrophages of SD rat either induced by DMBA or normal. It was higher than the phagocytic and ROI secretion activity of SD rat peritoneal macrophages induced by DMBA. It means that BCSHE extract enhanced the ROI secretion activity peritoneal macrophages induced by DMBA or not.
Activity test of black cumin seed hexane extract against macrophage phagocytic activity
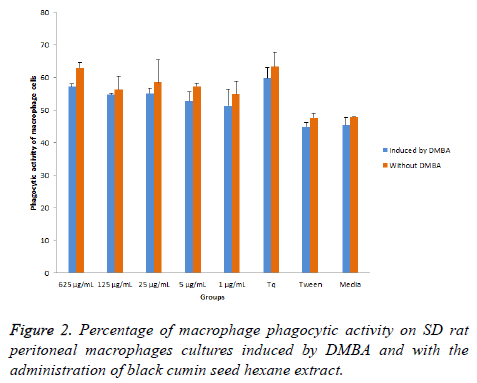
Phagocytic activity of SD rat macrophages induced by DMBA is showed in Figure 2, including the phagocytic activity on SD rat peritoneal macrophages culture induced by DMBA or without DMBA with the administration of black cumin seed hexane extract. The result showed that BCSHE could increase phagocytic activity in-vitro. Phagocytic activity of SD rat peritoneal macrophages without DMBA was higher than those of induced by DMBA. In general, BCSHE extract improve the phagocytic activity of macrophages induced by DMBA or not.
Discussion
The extraction methods and solvent substances determined the bioactive compound that can be extracted from black cumin seed hexane extract (BCSHE). Maceration method with hexane solvent followed by steam distillation produced an essential extract containing thymoquinone with the highest percentage of unsaturated fatty acid as 2.72% and the lowest percentage of unsaturated fatty acid was 24%. It also had 69% oleic acid. The previous study reported that the fatty extract of BCSHE mostly contains unsaturated fatty acids [9]. In addition, it also consists of triacylglycerol structure with typical triasil glycerol consists of 3 linolenic acid (LLL), 2 linolenic acid and 1 linoleic acid (LLO), 2 linolenic acid and one of palmitic acid (LLP), 1 linolenic acid and 2 linoleic acid (LOO) and 1 linolenic acid, 1 linoleic acid and 1 palmitic acid (LOP), which has a beneficial pharmacological effect to health. It also rich in sitosterol with beta-sitosterol as the main content. In 100 g of BCSHE contains 1 g of sterol, consist of 59.1% b-sitosterol, 16.5% stigmasterol, 14.4% isofucosterol and 10% kaempsterol.
The previous researcher has reported that the largest sitosterol content of BCSHE in Iran and Tunisia is beta-sitosterol (44-54%) and stigmasterol (11-20%) [6].
Sitosterol is a collection of plant sterol compounds that has various beneficial biological effects for health including antioxidant, antidiabetic, anti-hypercholesterolemia, and chemo-preventive [10,11]. B-sitosterol showed the chemopreventive effect on colon cancer by inhibiting lipid peroxidation in mice induced dimetilhidrazin [10,12]. Paniagua-Pérez (2008) revealed that it also protected cell damage from genotoxic and stimulate lymphocyte activity [13]. Six percents of black cumin seed essential extract is a ketone group monoterpenoid, consisting of karfon (4%), fenkon (1.1%), thymoquinone (0.6%) and dihidrokarfon (0.3%). The content of black cumin seed essential extract is nonterpenoid hydrocarbon group (4%), hydrocarbon monoterpenoid (26%), alcohol monoterpenoid (2.7%), hydrocarbon sesquiterpenoids (1%) and phenylpropanoid compound (64.1%) [14]. Although the content of thymoquinone in the black cumin seed volatile extract is low, it determines the pharmacodynamic effect of black cumin seed extract.
Nigellone is polymerized form of thymoquinone that can inhibit enzyme cyclooxygenase and lipoxygenase activity on the arachidonic metabolism, thus, might be used as an analgesic, anti-inflammatory and anticancer [15-17].
Macrophages are phagocytes that act as antigen presenting cell (APC) and effectors on natural and adaptive cellular immune response [18]. Research data showed that DMBA induction reduced the number and activity of peritoneal macrophages, phagocytic activity, and ROI secretion. In line with the results of this study, Torroella-Kouri et al. reported that DMBAinduced mice have a lower amount, less active and less reactive peritoneal macrophages [3]. It was associated with low expression of NFκB transcription factor and low level of E2 prostaglandin [3]. Exposure to DMBA has shown to be immunosuppressive and caused toxicity to the bone marrow [19], decreased the number of lymphocytes and inhibited spleen proliferation [20], iNOS and NO production by macrophages, and immunotoxic to macrophages [21].
Aside from thymoquinone, some unsaturated fatty acids such as sitosterol and terpenoids have also shown to increase the activity of macrophage phagocytosis and ROI secretion, which was exposed by xenobiotic. This is done through a cytoprotective mechanism by increasing the secretion of glutathione antioxidant by glutathione S-transferase (GST) due to the activation of the antioxidant responsive element (ARE). Results showed that the hexane extracts of black cumin seeds suppressed the activity of CYP genes and stimulated the activity of GST genes and enzymes.
Previous studies proved that black cumin seed extract served as a stimulant of phase II enzymes, both in vitro and in vivo assays [22,23]. Black cumin seed extract and thymoquinone serve as chemo-preventive through cytoprotective antioxidant effects by suppressing the activity of CYP genes (phase I) and increasing the activity of GST genes (Phase II) through Nrf2 activation, resulting in an increased production of GST enzyme for the detoxification process. Nrf2 gene is a gene that is responsible for oxidative stress, along with ARE [24,25].
Conclusion
Black cumin seed hexane extract (BCSHE) contained thymoquinone and unsaturated fatty acids. Hexane improves ROI secretion activity of SD rat’s peritoneal macrophages either DMBA-induced or without DMBA. Further research to assess the effectiveness of hexane as an in vivo antihepatotoxic immunomodulator along with acute and sub-chronic toxicity test is necessary to be done as a foothold for the development of drugs administration.
Acknowledgement
The research was funded by Dikti through the 2015 and 2016 competitive research grant.
References
- Randhawa MA, Al-Ghamdi MS. Anticancer activity of Nigella sativa (black seed)-A review. Am J Chin Med 2011; 39: 1075-1091.
- Akrom, Khoiri N, Suhana Y, Mustofa. Pengaruh pemberian ekstrak etanol biji Jinten hitam (N.sativa Lour) terhadap aktivitas fagositosis dan sekresi ROI makrofag mencit jantan galur Balb C secara in vitro, Seminar Nasional Tanaman Obat dan Obat Tradisional, Balai Besar Penelitian dan Pengembangan Tanaman Obat dan Obat Tradisional Badan Litbang Kesehatan, Departemen Kesehatan RI, Solo, 2007.
- Torroella-Kouri M, Silvera R, Rodriguez D, Caso R, Shatry A, Opiela S, Ilkovitch D, Schwendener RA, Iragavarapu-Charyulu V, Cardentey Y, Strbo N, Lopez DM. Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Res 2009; 69: 4800-4809.
- Akrom, Wijaya A. Effect of the ethanolic extract of Nigella sativa on phagocytosis activity of macrophage male swiss mice during infection of Listeria monocytogenes, The 4th International Eijkman Conference, Bali, 2007.
- Finlay TM, Jayanth P, Amith SR, Gilmour A, Guzzo C, Gee K, Beyaert R, Szewczuk MR. Thymoquinone from nutraceutical black cumin oil activates Neu4 sialidase in live macrophage, dendritic, and normal and type I sialidosis human fibroblast cells via GPCR Galphai proteins and matrix metalloproteinase-9. Glycoconj J 2010; 27: 329-348.
- Cheikh-Rouhou S, Besbes S, Lognay G, Blecker C, Deroanne C, Attia H. Sterol composition of black cumin (Nigella sativa L.) and Aleppo pine (Pinus halepensis Mill.) seed oils, J Food Comp Anal 2008; 21: 162-168.
- Akrom, Nurani LN, Hidayati T. Kajian aktivitas imunomodulator agen kemopreventif isolate aktif ekstrak N.sativa pada kanker payudara akibat paparan DMBA pada tikus putih, Laporan Penelitian, Yogyakarta LPP UAD 2008.
- Wijayanti MA. Sekresi tumor necrosis factor dan reactive oxygen intermediates oleh makrofag peritoneum mencit yang distimulasi dengan antigen terlarut Plasmodium falciparum. Berkala Ilmu Kedokteran 1997.
- Hassanein MMM, Abdel-Razek AG, Rudzinska M, Siger A, Ratusz K, Przybylski. Phytochemical contents and oxidative stability of oils from non-traditional sources. Eur J Lipid Sci Technol 2014; 116: 1-9.
- Baskar AA, Al Numair KS, Gabriel Paulraj M, Alsaif MA, Muamar MA, Ignacimuthu S. β-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer, J Med Food 2012; 15: 335-343.
- Gao J, Lauer FT, Dunaway S, Burchiel SW. Cytochrome P450 1B1 is required for 7,12-dimethylbenz(a)-anthracene (DMBA) induced spleen cell immunotoxicity. Toxicol Sci 2005; 86: 68-74.
- Baskar AA, Ignacimuthu S, Paulraj GM, Al Numair KS. Chemopreventive potential of β-sitosterol in experimental colon cancer model - an In vitro and In vivo study, BMC Comp Alt Med 2010; 10: 24.
- Paniagua-Pérez R, Madrigal-Bujaidar E, Reyes-Cadena S, Alvarez-González I, Sánchez-Chapul L, Pérez-Gallaga J, Hernández N, Flores-Mondragón G, Velasco O. Cell protection induced by beta-sitosterol: inhibition of genotoxic damage,stimulation of lymphocyte production, and determination of its antioxidant capacity, Arch Toxicol 2008; 82: 615-622.
- Nickavar B, Mojab F, Javidnia K, Amoli MA. Chemical composition of the fixed and volatile oils of Nigella sativa L. from Iran. Z Naturforsch C 2003; 58: 629-631.
- Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol:role of nuclear factor kB, cyclooxygenase 2, and matrix metalloprotease 9, Cancer Res 2002; 62: 4945-4954.
- Basu GD, Pathangey LB, Tinder TL, Lagioia M, Gendler SJ, Mukherjee P. Cyclooxygenase-2 inhibitor induces apoptosis in breast cancer cells in an in vivo model of spontaneous metastatic breast cancer. Mol Cancer Res 2004; 2: 632-642.
- Baldi A, Piccolo MT, Boccellino MR, Donizetti A, Cardillo I, La Porta R, Lucio Quagliuolo, Spugnini EP, Cordero F, Citro G, Menegozzo M, Cologero RA, Crispi S. Apoptosis induces by piroxicam plus cisplatin combined treatment is triggered by p21 in mesothelioma. PloS ONE 2011; 6: e23569.
- Charles Janeway. Janeway’s Immunology. Garland Science, 2012, New York.
- Page TJ, O’Brien S, Holston K, McWilliams PS, Jefcoate CR, Czuprynski CJ. 7,12-dimethylbenz[a]anthracene-induced bone marrow toxicityis p53-dependent, Toxicol Sci 2003; 74: 85-92.
- Gao J, Mitchell LA, Lauer FT, Burchiel SW. p53 and ATM/ATR regulate 7,12-dimethylbenz[a]anthracene-induced immunosuppression. Mol Pharmacol 2008; 73: 137-146.
- Gao J, Lauer FT, Mitchell LA, Burchiel SW. Microsomal expoxide hydrolase is required for 7,12-dimethylbenz[a]anthracene (DMBA)-induced immunotoxicity in mice. Toxicol Sci 2007; 98: 137-144.
- Padhye S, Banarjee S, Ahmad A, Mohammad R, Sarkar FH. From here to eternity-the secret of pharaos: Therapeutic potential of black cumin seeds and beyond. Cancer Ther 2008; 6: 495-510.
- Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol 2005; 5: 1749-1770.
- Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap 1, -Nrf2 pathway, Arch Toxicol 2011 85: 241-272.
- Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr 2001; 131: 3027S-3033S.