Research Article - Biomedical Research (2020) Volume 31, Issue 5
Evaluation of antibacterial and non-antibacterial adhesives in terms of cytotoxicity in cell culture medium
Derya Turel Silsupur, Elif Pinar Bakir*, Seyhmus Bakir
Department of Restorative Dentistry, Dicle University, Diyarbakir, Turkey
- Corresponding Author:
- Elif Pinar Bakir
Department of Restorative Dentistry
Dicle University
Diyarbakir
Turkey
Accepted date: October 08, 2020
Abstract
Purpose: The purpose of the study is to evaluate the adhesive systems containing antibacterial components (Clearfil SE Protect Bond-CPB, Peak Unıversal Bond-PUB, Gluma 2 Bond-G2B) and adhesive systems without antibacterial components (Clearfil SE Bond-CB, Gluma Self Etch Bond-GB) in terms of cytotoxicity in the cell culture medium.
Materials and methods: We compared the cytotoxic effects of five different adhesive systems in the cell culture medium by direct contact method. Four different dilutions (1%, 0.1%, 0.01%, 0.001%) of test materials were incubated in three different periods (24-48-72 hours) in the L929 mouse fibroblast cell culture medium and their effects on cell proliferation were evaluated with the XTT Tetrazolium Assay test.
Results: Comparing the cytotoxic effects of five different adhesive systems as time-dependent, the two highest concentrations of CB and CPB and only the highest concentration of GB showed significant cytotoxicity (P<0.05) in the 24-hour incubation. PUB showed cytotoxic effects at all concentrations tested. This effect has been shown to be dose-dependent. In 48-hour and 72-hour incubations, GB and PUB increased the cytotoxic effect in a dose-dependent manner (P<0.05). On the other hand, G2B, CB and CPB were found to be cytotoxic only at the two highest concentrations and the difference of CPB was more significant (P<0.001).
Conclusion: Antibacterial contents added to adhesive systems had no adverse effect on biocompatibility. The highest concentration caused the highest cytotoxic effect in adhesive systems with and without antibacterial components, which increased with incubation time.
Keywords
Antibacterial Adhesives, Cytotoxicity, XTT
Introduction and Purpose
With the increasing health awareness in society, the expectations of individuals from dentists have increased. New materials are produced and presented to the market in the field of dentistry. This situation requires the examination and evaluation of the physical, chemical, and biological properties of these materials. The minimally invasive technique that minimizes substance loss in teeth has become feasible with the development of adhesive systems. The minimally invasive method that minimizes tooth substance loss has become possible with the development of adhesive systems [1].
Total-etch adhesive systems are based on the demineralization of dental tissue with 37% phosphoric acid and then applying primer and then adhesive resin on the rough surface formed on the tooth surface. With the polymerization of adhesive resin with light, resin tags are formed, and micro-mechanical bonding occurs between the tooth and the adhesive system [2]. A resistant hybrid layer is formed between the penetration of monomers and subsequent polymerization between the collagen fibrils released by acid application. Three-step systems are considered a gold standard. However, clinicians' desire to save time and effort has resulted in the development of single-bottle systems, in which adhesive and primer are collected in a single solution [3-7].
Self-etch adhesive systems are classified as two- step and single-step according to the number of steps and strong, moderate and mild based upon pH-degrees. In recent years, single-step “all-in-one” self-etch systems have been produced that combine three application steps (Acid, primer and adhesive resin) to provide ease of application.
Self-etch adhesive systems are evaluated in three groups as strong (pH<1), moderate (1 ≤ pH ≤ 2), and mild (pH>2) based upon their pH-degrees. Total- etching adhesive systems are recommended to be used in superficial cavities and self-etching adhesive systems in young, deep and highly permeable cavities. Adhesives are composed of different components with functions such as resin monomers (HEMA, BisGMA, TEGDMA and UDMA), solvents, inhibitors, initiators and filler particles [3,4,6-8].
It is expected that an ideal restorative material will show strong adhesion to the dental tissues, prevent bacterial transmission between the restorative material and the cavity wall, and prevent micro-leakage, which may cause complications such as post-operative sensitivity, marginal coloration, secondary caries and ultimately pulp inflammation and necrosis. Despite the progress made in restorative materials and adhesive systems, it has not been possible to prevent microleakage completely. Therefore, precautions should be taken to prevent microorganisms from invading the tooth-restoration material interface. In this context, creating an antibacterial effect by adding antibacterial agents to adhesive systems is an innovative and promising approach [4,9-12].
Antibacterial components added to self-etch adhesive systems
Methacryloxy dodecyl pyridinium bromide (MDPB): Methacryloxy dodecyl pyridinium bromide (MDPB), which is one of the antibacterial components used to add disinfectant properties and to act on the bacteria in the dentin canal, was synthesized from a methacrylate group and quaternary ammonium, an antibacterial agent [13]. The quaternary ammonium compounds that cationically bind to the cell wall disrupt membrane function and cause the cytoplasmic material to leak out. They cause the lysis of bacterial cells by showing a strong bactericidal effect. It is reported that MDPB provides a bactericidal effect without any side effects on the biocompatibility of resin- based dental material to which it is added [10].
Chlorhexidine: Chlorhexidine, which was produced as a synthetic chemotherapeutic agent in the 1940s, is used in the form of chlorhexidine digluconate for the chemical control of dental plaque and prevention of dental caries. Chlorhexidine is a bis-biguanide compound and has a quaternary ammonium structure. It has broad- spectrum antibacterial activity and has a bacteriostatic and bactericidal effect on the gram (+), less gram (-) facultative anaerobe and aerobe microorganisms. Chlorhexidine, a matrix metalloproteinase (MMP) inhibitor, has been reported to prevent collagen collapse, increasing the durability of the adhesive system and extending the life of adhesive restorations as a result of suppressing MMPs in the hybrid layer [14,15].
Glutaraldehyde: Another compound with an antibacterial effect is glutaraldehyde. It binds strongly to the carboxyl, hydroxyl sulfhydryl and amino groups of the microorganisms and its outer layer and interacts with the cell wall of gram-negative and gram-positive bacteria. It prevents the cross-linking of amino acids in proteins by blocking the transport processes of bacteria. It inhibits dehydrogenase activity and permeases and prevents RNA, DNA and protein synthesis [5,16].
When adding antibacterial components to adhesive systems, the tissue response, and the possibility of toxic effects, in other words, the biocompatibility of the component should be investigated. Biocompatibility is that a material does not produce local and systemic toxicity or tissue reactions such as allergic, immunogenic, mutagenic and carcinogenic effects as a result of contact with living tissue [3,17,18]. Three steps should be followed in the evaluation of biocompatibility: in vitro, in vivo (animal testing) and use testing [3,15,17,19,20].
In vitro testing
In cell culture studies, which is an in vitro test method in which the biocompatibility of restorative materials is evaluated, the effect of the experimental material on the viability, morphology, growth rate, cellular functions, membrane permeability, and damage and various enzyme activities are evaluated with vitality, life, cell proliferation and metabolic cytotoxicity assessment tests [17,19,20].
Cell culture tests have advantages such as direct observation of the effects of the material on the cells and the repeatability of the processes. There are, however, some disadvantages: environmental conditions can affect the outcome of the experiment, it is a costly method, and cells may differ or die in successive passages after primer culture. In the direct contact test, the material is placed directly on the cells in the culture, and the dose-response curve determines which components of the material are toxic. Colorimetric, luminescence and enzymatic methods can be used to assess cell viability in cytotoxicity tests. Among the colorimetric methods, assays such as XTT and MTT, which are performed with tetrazolium salts, are most frequently used.
XTT assay
XTT sodium salt (sodium 3′-[1-[(phenylamino)-carbony]- 3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene-sulfonic acid hydrate) is a tetrazolium salt that is actively absorbed into cells. XTT is reduced to orange colored water- soluble formazan compounds as a result of biodegradation reactions by mitochondrial dehydrogenase enzymes in cells with metabolic activity. Only mitochondria in living cells can reduce the XTT salt and turn it into an orange-colored water-soluble dye. The density of the dye is measured with the spectrophotometer device and at the specified wavelength (450 nm-500 nm). The cell lines most frequently used in cytotoxicity tests are continuous cell lines such as primary cells or L-929 mouse fibroblast cell, BALB/3T3 mouse embryo fibroblasts, MDPC-23 mouse odontoblast cells [7,18,20]. The cell culture used should be produced from the type of tissue in which the material will be used in the human body. Since it is difficult to obtain a dental pulp, L 929 cells (mouse fibroblast cell), which are the connective tissue cells closest to the pulp, are preferred in studies.
The biocompatibility of dental adhesive systems depends on factors such as the area of application, the distance to the pulp, the concentrations of the components and their interactions with each other, the type of light device, the application time of the light, the contact time and the host response [21]. The adhesive system cannot be used in experimental animals and then in humans unless it passes in vitro cytotoxicity tests. The fact that the application area of dental adhesive systems is close to the pulp tissue and also the possibility of contamination of the gum and oral mucosa necessitated further studies on this subject [7,22,23].
Animal testing
Dental materials must be used in experimental animals before use tests.
In animal experiments, where mammals such as mice and rats are used, there are different parameters such as the form of the material, the contact time and delivery method of the material, the type, age, and sex of the animals. Local and systemic toxic effects of dental material are determined by macroscopic and microscopic examination of the tissues at different periods after implantation (subcutaneous, intramuscular or intraosseous) into the tissue [3,19,20].
Use tests
Use tests (Clinical Studies) are based on the observation of the effects of a material found reliable in in vitro and in vivo tests on the human body. Tissue response parameters such as pulp reactions, gingiva, and periodontium, inflammation in the oral mucosa, apoptosis and necrosis are evaluated in clinical studies of biocompatibility of dental materials. It was aimed in this study to determine the cytotoxic effect of three different self-etch adhesives (Peak universal bond, Clearfil protect bond, Gluma 2 bond) containing antibacterial agent (glutaraldehyde, chlorhexidine, and MDPB) and two different self-etch adhesives (Gluma self- etch bond, Clearfil SE Bond) without antibacterial agent using XTT assay by direct contact method.
Materials and Methods
In our study, the dose/incubation-time dependent cytotoxic effect of five different adhesive materials (Table 1) routinely used in the clinic was evaluated with XTT assay per ISO criteria. Microscopic studies were carried out at Selcuk University Advanced Technology Research and Application Center. As a result of mixing the primer and adhesive, some components can interact with each other and affect the test result. To prevent this situation, only the primaries of the two-step adhesive systems were included in the study.
| Adhesives | Manufacturer | Lot number |
Chemical ingredient |
|---|---|---|---|
| Gluma self etch bond (GB) |
Heraus Kulzer,Hanau,Germany | 010912 | UDMA(Urethane dimethacrylate ),4-MEc: TA/Acidic monomer, acetone, water fillers,photoinitiators, stabilizers |
| Gluma 2 bond (G2B) |
Bayer AG,Germany | 010512 | Ethanol, 2-hydroxyethyl methacrylate, poly (methacrylic-oligo-acrylic acid), 4-methacryloxyethyltrimellitic acid anhydrad, glutaral, amorphous silica |
| Clearfil SE bond (CB) |
Kuraray, Okayama, Japonya |
Primer lot no 450247 |
Primer:2- hydroxyethylmethacrylate, 10-methacryloyloxydecyl dihydrogen phosphate, hydrophilic aliphatic dimethacrylate, camferoquinone, water, accelerators Bond:Bis-Gma, Hema, Hydrophobic dimethacrylate, camphorquine,N-N-Diethanol-p-toluidine,colloidal silica |
| Clearfil SE Protect bond (CPB) |
Kuraray, Okyama, Japonya |
Primer lot no 3E0068 |
Primer:2- hydroxy ethyl methacrylate, 10-methacryloyloxydecyl dihydrogen phosphate, hydrophilic aliphatic Bond:Bis-Gma, Hema, Hydrophobic dimethacrylate, camphorquine,N-N-Diethanol-p-toluidine,colloidal silica |
| Peak universal bond (PUB) |
Ultradent, Utah, USA | 010912 | Ethyl alcohol, 2-hydroxyl methacrylate, methacrylic acid, 0.2% chlorhexidine |
Table 1: Characteristic features of the adhesives used.
Preparation of test materials
The main stock solutions of 1%, in which the materials were dissolved in Dulbecco’s Modified Eagles Medium (DMEM), were prepared, and the solutions were sterilized by passing them through 0.2 μm sterile filters. Test dilutions (0.1%, 0.01% and 0.001%) were prepared by diluting the samples taken from these stock solutions with a serial dilution of 1/10 in DMEM three times. As a result, four different doses of test materials were used in the analysis of cytotoxicity. The wells containing cells from which test materials were not added were used as the control group.
Cell culture analysis
L929 mouse fibroblast cells were produced in DMEM medium (containing 10% fetal bovine serum and 100 U/ ml penicillin-100μg/ml streptomycin), T75 cell culture plates, at 37°C and in a 95% humidified drying-oven containing 5% CO2. When the adhesive cells covered 75-80% of the cell culture plate, they were removed from the flask base using the trypsin enzyme. 100 μl homogenized cell suspension was diluted 1:1 with 0.04% trypan blue. The number of cells in 1 ml was calculated by a hemocytometer.
XTT cell proliferation and viability assay
Sterile flat-bottom 96-well plates were used in the XTT method. 10×103 L929 cells were planted in 100 μl of a medium in each well and incubated overnight in a 95% humidified oven with 5% CO2 at 37°C. The next day, cells that reached about 75% confluence were removed from the medium just before adding the substance. Suspensions containing different doses (1%, 0.1%, 0.01% and 0.001%) of samples prepared by serial dilution in DMEM were added to the determined wells in a volume of 100 μl. The wells containing no substance and only cells were designated as negative control (cell control) group, and the wells containing only medium and no cell and substance (blank: medium control) and only the cell-containing wells (substance control) were control groups. For each adhesive system, cells were incubated in a 95% humidified oven with 5% CO2 at 37°C in 3 different periods: 24 hours, 48 hours and 72 hours. After incubations, cells were examined morphologically at x10 magnification with an inverted microscope(Table 1). Then, a cell proliferation test was performed with the XTT kit. The XTT solution was prepared in a sterile environment by mixing 5 ml solution A (XTT solution) and 100 μl solution B (activator solution) for one 96-well plate according to the kit protocol. XTT solution (50 μl/well) was added to each well. The plates were kept at 37°C for at least 4-5 hours. After incubation, the optical density (OD) of the chromogenic product was measured with a microplate reader (Epoch, BioTek, USA) at a wavelength of 460 nm (reference wavelength 650 nm). Ten wells were used for each dilution of each test material and experiments were repeated twice. The microplates created for XTT assay were subjected to morphological examinations of the cells after incubations at x10 magnification with an inverted microscope. Then, a cell proliferation test was performed with the XTT kit. Cytotoxic activities of five different dental adhesives were detected with L929 cells in four different dilutions (0.001%, 0.01%, 0.1% and 1%) and at the end of three different incubation periods (24, 48 and 72 hours).
Statistical analysis
Bidirectional variance analysis was applied in repeated measurements to evaluate the effect of different doses and incubation periods of the applied test materials on cell viability. Bonferroni post-test was applied to determine which factor and the impact caused the result in the groups found significant (P: significance level) (values are significant for ***: P<0.0001**: P<0.01*: P<0.05*. Data for P>0.05 values are not statistically significant.)
Findings
In the study, the cytotoxicity of Gluma Self Etch and Clearfil SE Bond adhesive systems, which have a similar composition with Gluma 2 Bond (glutaraldehyde) Clearfil SE Protect (MDPB) and Peak Universal Bond (chlorhexidine) adhesive systems with antibacterial effect, yet without antibacterial content, were evaluated with XTT assay. The mean of the viability values (%) obtained as a result of the dose and incubation period of the test materials are shown in Table 2 along with their standard deviations.
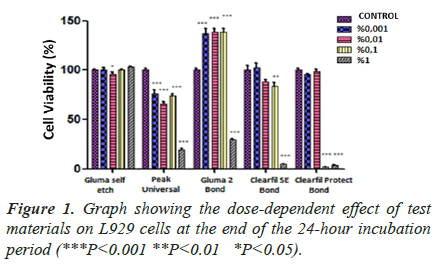
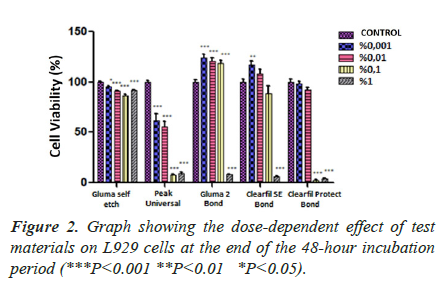
Dose-dependent effect of adhesive systems at the end of 24, 48 and 72 hours incubation periods (Figures 1-3).
| Interaction Groups |
Gluma self-etch | Peak Universal | Gluma 2 Bond | Clearfil SE Bond | ClearfilProtect Bond | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| t | P | t | P | t | P | t | P | t | P | |
| Control/ %0.001 | ||||||||||
| 24-hour | 0.1719 | P>0.05 | 4.054 | P<0.001*** | 9.201 | P<0.001*** | 0.4681 | P>0.05 | 2.398 | P>0.05 |
| 48-hour | 2.730 | P<0.05* | 6.527 | P<0.001*** | 5.940 | P<0.001*** | 3.289 | P<0.01** | 0.7237 | P>0.05 |
| 72-hour | 3.463 | P<0.01** | 1.692 | P>0.05 | 6.079 | P<0.001*** | 3.023 | P<0.01** | 0.9037 | P>0.05 |
| Control/%0.01 | ||||||||||
| 24-hour | 2.463 | P<0.05* | 5.821 | P<0.001*** | 9.428 | P<0.001*** | 2.193 | P>0.05 | 0.5132 | P>0.05 |
| 48-hour | 5.461 | P<0.001*** | 7.646 | P<0.001*** | 5.198 | P<0.001*** | 1.487 | P>0.05 | 4.100 | P<0.01** |
| 72-hour | 11.50 | P<0.001*** | 5.316 | P<0.001*** | 8.038 | P<0.001*** | 3.124 | P<0.01** | 1.421 | P>0.05 |
| Control/%0.1 | ||||||||||
| 24-hour | 0.06122 | P>0.05 | 4.431 | P<0.001*** | 9.516 | P<0.001*** | 3.012 | P<0.01** | 53.84 | P<0.001*** |
| 48-hour | 7.891 | P<0.001*** | 15.78 | P<0.001*** | 4.634 | P<0.001*** | 2.187 | P>0.05 | 53.55 | P<0.001*** |
| 72-hour | 14.21 | P<0.001*** | 14.66 | P<0.001*** | 6.154 | P<0.001*** | 0.2782 | P>0.05 | 54.51 | P<0.001*** |
| Control/%1 | ||||||||||
| 24-hour | 1.879 | P>0.05 | 13.82 | P<0.001*** | 17.38 | P<0.001*** | 17.92 | P<0.001*** | 52.88 | P<0.001*** |
| 48-hour | 5.053 | P<0.001*** | 15.48 | P<0.001*** | 22.63 | P<0.001*** | 17.68 | P<0.001*** | 53.01 | P<0.001*** |
| 72-hour | 12.94 | P<0.001*** | 16.05 | P<0.001*** | 23.05 | P<0.001*** | 17.92 | P<0.001*** | 54.35 | P<0.001*** |
| %0.001/%0.01 | ||||||||||
| 24-hour | 2.635 | P<0.05* | 1.766 | P>0.05 | 0.2267 | P>0.05 | 2.662 | P<0.05* | 1.885 | P>0.05 |
| 48-hour | 2.732 | P<0.05* | 1.119 | P>0.05 | 0.7425 | P>0.05 | 1.803 | P>0.05 | 3.376 | P<0.01** |
| 72-hour | 8.037 | P<0.001*** | 3.623 | P<0.01** | 1.960 | P>0.05 | 0.1015 | P>0.05 | 2.324 | P>0.05 |
| %0.001 vs %0.1 | ||||||||||
| 24-hour | 0.2331 | P>0.05 | 0.3772 | P>0.05 | 0.3147 | P>0.05 | 3.480 | P<0.01** | 51.44 | P<0.001*** |
| 48-hour | 5.162 | P<0.001*** | 9.248 | P<0.001*** | 1.306 | P>0.05 | 5.476 | P<0.001*** | 52.83 | P<0.001*** |
| 72-hour | 10.74 | P<0.001*** | 12.96 | P<0.001*** | 0.07562 | P>0.05 | 2.745 | P<0.05* | 53.60 | P<0.001*** |
| %0.001 vs %1 | ||||||||||
| 24-hour | 1.707 | P>0.05 | 9.770 | P<0.001*** | 26.58 | P<0.001*** | 18.39 | P<0.001*** | 50.48 | P<0.001*** |
| 48-hour | 2.324 | P>0.05 | 8.957 | P<0.001*** | 28.57 | P<0.001*** | 20.97 | P<0.001*** | 52.29 | P<0.001*** |
| 72-hour | 9.472 | P<0.001*** | 14.36 | P<0.001*** | 29.13 | P<0.001*** | 20.94 | P<0.001*** | 53.45 | P<0.001*** |
| %0.01 vs %0.1 | ||||||||||
| 24-hour | 2.402 | P>0.05 | 1.389 | P>0.05 | 0.08794 | P>0.05 | 0.8187 | P>0.05 | 53.33 | P<0.001*** |
| 48-hour | 2.430 | P<0.05* | 8.129 | P<0.001*** | 0.5638 | P>0.05 | 3.674 | P<0.001*** | 49.45 | P<0.001*** |
| 72-hour | 2.705 | P<0.05* | 9.341 | P<0.001*** | 1.884 | P>0.05 | 2.846 | P<0.05* | 55.93 | P<0.001*** |
| %0.01 vs %1 | ||||||||||
| 24-hour | 4.342 | P<0.05* | 8.004 | P<0.001*** | 26.81 | P<0.001*** | 15.72 | P<0.001*** | 52.37 | P<0.001*** |
| 48-hour | 0.4080 | P>0.05 | 7.838 | P<0.001*** | 27.83 | P<0.001*** | 19.16 | P<0.001*** | 48.91 | P<0.001*** |
| 72-hour | 1.435 | P>0.05 | 10.73 | P<0.001*** | 31.09 | P<0.001*** | 21.04 | P<0.001*** | 55.77 | P<0.001*** |
| %0.1 vs %1 | ||||||||||
| 24-hour | 1.940 | P>0.05 | 9.393 | P<0.001*** | 26.89 | P<0.001*** | 14.91 | P<0.001*** | 0.9628 | P>0.05 |
| 48-hour | 2.838 | P<0.05* | 0.2909 | P>0.05 | 27.27 | P<0.001*** | 15.49 | P<0.001*** | 0.5396 | P>0.05 |
| 72-hour | 1.270 | P>0.05 | 1.392 | P>0.05 | 29.20 | P<0.001*** | 18.20 | P<0.001*** | 0.1555 | P>0.05 |
t: t value, P: significance level (***: p<0.0001, **: p<0.01, *: p<0.05 * values are significant. Data at P>0.05 values are not statistically significant.)
Table 2: Bonferroni test table of XTT assay results.
In the GB group, there is a significant difference (P<0.001***) between both dose and incubation periods and dose-incubation period interaction groups. It is clear that the increase in dose and incubation period in L929 cells significantly reduces the % viability rate. However, the fact that the cell viability did not fall below 50% in any incubation period, even at the highest dose tested, showed that the toxic effect of GB was very low. In the 24-hour incubation, the viability does not decrease significantly as the dose increases. On the other hand, in 48-hour and 72-hour incubation, only high doses of material appear to significantly reduce cell viability compared to control.
In the PUB group, there is a significant difference (P<0.001***) between both dose and incubation periods and dose-incubation period interaction groups (Table 2). For all incubation periods, it is seen that all doses tested reduce cell viability significantly compared to control (P<0.001***). In 24-hour incubation, it was found that cell viability did not significantly change as doses decreased. In 48- and 72-hour incubation periods, it is seen that there is a statistically significant difference between 0.01% and 0.1% doses, but the difference in other doses is not significant. As a result, it is seen that all doses of PUB significantly reduced cell viability for all incubation periods, compared to the control group.
In the G2B group, there is a significant difference (P<0.001***) between both dose and incubation periods and dose x incubation time interaction groups. In the 24- hour, 48-hour, and 72-hour incubation, as a result of the comparison made based on dose groups, the viability does not decrease significantly as the dose increases. The material significantly reduces cell viability compared to control at only the highest dose of 1% concentration. This decrease increases in a time-dependent manner. It is noteworthy that the cell viability does not decrease from 0.1% dose and even increases statistically significantly compared to the control. G2B significantly reduces cell viability at only the highest dose of 1% concentration, compared to the control group. This decrease increases in a time-dependent manner.
While there is a significant difference (P<0.001***) between dose and incubation periods in the CB group, the difference between the dose-incubation period interaction groups is less significant (P<0.05**). For all incubation periods, it is clear that the material significantly reduces cell viability compared to control at the highest dose (1% concentration) tested (P<0.001***). However, at lower doses of the material, it was found that it did not significantly reduce cell viability compared to control, or even increased at the lowest two doses (P<0.01). Besides, the reduction is not time-dependent in 1%, the only dose that causes a statistically significant decrease in cell viability. As a result, it is clearly seen that CB significantly reduces cell viability for all incubation periods, compared to the control group, at the highest dose (1% concentration) of the material.
In the CPB group, there was no significant difference in cell viability between doses (P<0.001***), while no significant difference was observed between incubation periods (P=0.4023). However, there was a significant difference (P<0.001***) between dose x incubation time interaction groups. For all incubation periods, it is clear that only 1% and 0.1% doses significantly reduce cell viability compared to the control (P<0.001***). In all incubation periods, 0.01% and 0.001% doses do not make a statistically significant difference (P>0.05) in cell viability, both from each other and from the control, regardless of the incubation period. It is clear that for all incubation times of CPB, only 1% and 0.1% doses significantly reduce cell viability compared to the control group.
Time-dependent effect of adhesive systems at the end of 24-, 48- and 72-hour incubation periods
In the 24-hour incubation, the highest two concentrations of CB and CPB, and only the highest concentration of GB, had a high cytotoxic effect (P<0.05). It was observed that GB had no cytotoxic effect on cells (P˃0.05). In contrast, PUB showed a significant impact at all concentrations tested (Figure 1). In both 48-hour and 72-hour incubation, only GB and PUB adhesives were shown to increase the cytotoxic effect in a dose-dependent manner (P<0.05). At the 48th and 72nd hour, the efficacy of PUB, which was observed to be cytotoxic even at its lowest concentration, was found to be highly significant (P<0.001). On the other hand, only the highest concentrations (1%) of GB and CB materials were effective. The two highest concentrations (0.1% and 1%) of CPB material were found to make a significant difference (p<0.001) compared to control in both 48- and 72-hour incubation (Figures 2 and 3). As a result of these findings, it can be said that the five materials had similar effects, although their significance levels were different in both 48 and 72 hours incubation.
Discussion
New materials are produced and presented to the market in the field of dentistry. This situation requires the examination and evaluation of the physical, chemical, and biological properties of these materials. We preferred the in vitro test method in our study, considering the rapid results, low cost, standardization, control of the experimental environment and evaluation on a full scale. Cell culture tests are the most widely used in in vitro cytotoxicity test methods. According to the contact material applied in the culture with the test material applied, direct contact and extract test can be used, as well as an indirect contact test with a barrier in between [3,19]. In the indirect contact test, it is an advantage to use a barrier that simulates the remaining dentin tissue. However, direct contact testing is often preferred. Because, in the indirect contact test, dentin permeability varies, difficulties in forming the barrier test device are encountered and there is a high cost [19]. In the study, we used the direct contact test recommended for use in in vitro cytotoxicity tests within the scope of ISO 7405 and ISO 10993: 5 [24].
Adhesive systems do not come in direct contact with the pulp tissue. However, due to the approach to the pulp when cleaning caries, the diameter of the dentin tubules gradually increases, and therefore the pulp tissue is exposed to acute toxic effects of resin-containing systems. This toxic effect causes pulp necrosis, apoptosis (programmed cell death) and inhibition of healthy pulp development [17]. In our study, 1/100, 1/1000, 1/10000 serial dilutions obtained from adhesive systems were placed in the wells where the cells were incubated, so that the cells were contacted directly with the test material. This made it possible to eliminate the changes in the dentin permeability and to observe the cytotoxicity of the serial material dilutions of the adhesive material on the cells. It should be kept in mind that direct contact of cells and material results in higher value cytotoxic effects compared to clinical applications, in direct contact testing [3].
Immortal cell lines (HeLa, 3T3, and L 929) and primary/ diploid cell lines (gingival, mucosal and pulpal fibroblasts) are used in studies using an in vitro cell culture test.2,8 In our study, a clonal cell line was used, consisting of fibroblast-derived L929 mouse fibroblasts derived from mouse subcutaneous connective tissue. Thus, it is aimed to obtain precise results close to the effect on dental pulp cells.
Different methods can be used to determine the cytotoxic effects of test materials on cell cultures. The most commonly used of these methods are determining the number of remaining living cells, determining the proliferation rates of the cells, and examining the molecular synthesis or enzyme activity in the cells [19].
Dyes such as trypan blue, erythrosine, and naphthalene black, which enter the cell as a result of membrane integrity deterioration, or dyes such as diacetyl fluorescent and neutral red, which can enter the cell without disrupting the integrity of the membrane, are used in determining the number of living cells remaining in the cell culture. In determining cell viability, MTT, LDH and Alamar Blue assays are the primary tests that can distinguish changes in cell metabolism based on metabolic activity. MTT Assay is a high precision, cheap and fast method. However, dimethyl sulfoxide DMSO, which is preferred as a solvent to dissolve the formazan formed in MTT Assay, is toxic to cells. This reduces test reliability [6,18,25,26]. In XTT Assay, no additional dissolve operation is required. It is also possible to record the absorbance at various times during the experiment. In this way, XTT assays provide more detailed information about the evaluation of viability and more sensitive information about the number of cells compared to the MTT assays [27]. For this reason, XTT assay was used in cytotoxicity evaluation in our study.
Li et al. [28] evaluated the cytotoxicity of different concentrations (100%, 5%, and 25%) of five different adhesive materials (Super-Bond, Clearfil SE Bond, G-Bond, Single Bond2, and Adper Easy One) on human periodontal ligament cells (HPDLCs) at different time periods (24-, 48-, and 72-hour) by MTT assay. In the study, they explained the cytotoxic effects of the materials used as Super Bond <Clearfil SE Bond <G-Bond <Adper Easy One <Single Bond2. They stated that the cytotoxicity of the adhesives was affected by different concentrations (25%<50%<100%) and application periods (24<48<72 hours).
Chen et al. [29] investigated the cytotoxic effects of dentin bonding systems used in the ratio of 1/1000, 1/2000 and 1/4000 on pulp fibroblasts, and found the cytotoxic impact at the highest value in 1/1000 dilutions. As a result of in vitro cytotoxicity studies with dentine barrier tests, they concluded that the presence of >500 μm dentin layer is sufficient to protect the pulp and that systems with low pH do not cause pulp damage.
Some studies have reported that applying total-etching adhesive to deep cavities results in chronic inflammation and granulomatous reaction in the human pulp. A self- etch adhesive system are recommended for use in young, deep and permeable dentine, as they leave the smear layer and plugs that limit the diffusion of unpolymerized monomers towards the pulp. Therefore, we used the self- etch adhesives in the study.
It has been reported that dentin binding systems increase the effect of monomers in its content by prolonged contact with the dentin layer [30]. Therefore, we applied the test materials in three different periods (24,48 and 72 hours) in the study to evaluate the cytotoxic effects occurring in the short, medium and long term. We have concluded that the cytotoxic effect of the adhesive systems we use varies depending on time.
In their study in 2014, Cal et al. [29] evaluated the cytotoxic effects of five different dentin adhesives (Admira Bond, Adper Single Bond Plus, Clearfil SE Bond, Clearfil S3 Bond, and Heliobond) on human gingival fibroblast cells at different periods. They found that all materials had a serious cytotoxic effect with cell viability rates ranging from 6-24% within the first 24 hours. In another study, Chen et al. [29] examined the cytotoxic effects of the binding agents they diluted at different concentrations after 24 hours on pulp cells and reported that the cytotoxic effect increased as the concentration increased.
Only primer parts of two-step self-etch adhesives were applied to cell culture inserts in our study, to reflect the actual clinical situation. Adhesive resins of the systems are not included in the study to eliminate conditions that may affect the test result since some components will interact with each other as a result of mixing the primer and adhesive.
It was concluded that the cytotoxic effect of adhesive systems varies not only in a time-dependent manner but also in a dose-dependent manner [5]. Ratanasathien et al. [5] reported that cytotoxicity decreased as the concentration of the material decreased.
The cytotoxic monomers of these materials and their interactions with each other are also an essential factor in the cytotoxic effects of the materials [31]. Ratanasathien et al. [5] reported that UDMA had a higher cytotoxic effect than HEMA, and the interaction of the substances in their contents with each other, in addition to the concentration of binding agents, is also effective in cytotoxic effects.
Monomers such as TEGDMA and HEMA in adhesive systems can reach the pulp through dentin tubules and damage the pulp at high concentrations [32]. Resins can reach pulp through dentinal tubules after a while when they are not fully polymerized. Gerzina and Hume [10] have detected the presence of TEGDMA and HEMA, which pass through the dentine, reaching the pulpal tissue even a hundred days after polymerization.
Susgun et al. [6] evaluated qualitatively and quantitatively the cytotoxicity of different single-stage self-etch adhesive materials (Prime and bond one select, Optibond All-in-one, G-bond, Clearfil universal bond, Single bond universal) in the cell culture medium and found that SBU, CUB, GB, and OB-AIO have similar cytotoxic effects. Moreover, they determined that all the adhesive material tested had varying degrees of cytotoxic potential, varying depending on dose and time.
Adhesive systems cause cytotoxic effects not only on the pulp but also on the mucosa and the oral mucosa. In their study on gingival fibroblasts, Issa et al. [24] showed that the most cytotoxic effect on cells that have been in contact with the monomers for 24 hours is caused by Bis-GMA, followed by TEGDMA and HEMA, respectively. Many studies examining the cytotoxic effects of components in binding agents have concluded that Bis-GMA is the most toxic structure, followed by UDMA, TEGDMA, HEMA, and MMA, respectively [22,31].
The first of the adhesive systems containing the antibacterial agent used in our study is the two-step self-etch adhesive Clearfil Protect Bond (CPB) with a primer MDPB monomer. This adhesive system has an antibacterial effect, thanks to MDPB. In vitro studies on the subject reported that MDPB-containing CPB exhibits antibacterial properties in in vitro conditions [33,34]. In their research on the cuspids, Imazato et al. [33] found the antibacterial activity of CPB against S.mutans to be significantly successful.
In their studies comparing the cytotoxic effects of different dentin binder systems (Clearfil Protect Bond, Adper ScotchBond1, Xeno III, Prime & Bond NT), Grobler et al. [35] found that the dentin binder called Xeno III had the most toxicity, followed by CPB. The researchers stated that the primer part of the CPB was three times more toxic than the bond part and that the primer part containing the MDPB monomer could cause this.
In their study comparing the cytotoxic effect of dentin desensitizing agents with the dentin barrier test method, Wiegand et al. [36] found that the Clearfil Protect Bond dentin binding agent was significantly cytotoxic than other test materials. Researchers explained the cytotoxic effect of CPB with the MDPB monomer contained in this material [9,37]. In our study, CPB significantly reduced cell viability at only 1% and 0.1% doses for all incubation periods, compared to the control (p<0.001***). This decrease was independent of the incubation period.
Koulaouzidou et al. [22] compared the cytotoxicity of six different bonding agents using monolayer cell cultures (Admira Bond, Clearfil Liner Bond 2V ED Primer II, Fuji Bond LC, Gluma Comfort Bond, and NanoBond). Gluma Comfort Bond with Glutaraldehyde showed the highest cytotoxic effect on cell cultures according to the results of this study. According to researchers, glutaraldehyde is more cytotoxic than HEMA in monolayer cell cultures, and this result may have been due to the HEMA and glutaraldehyde content of Gluma Comfort Bond [10].
In their study using 10% polyvinyl pyrrolidone, 2% sodium hypochlorite, 2% glutaraldehyde, Keskin et al. [15] infected microorganisms on channel files, diamond burs and ultrasonic stone cleaner tips. According to the results of the study, 2% glutaraldehyde was found to be more effective compared to other disinfectants.
It is seen that Gluma 2 Bond (Heraeus Kulzer Gmbh, Hanau, Germany), which is the glutaraldehyde bond used in our study, does not significantly decrease vitality as the dose increases in 24-hour, 48-hour and 72-hour incubation. It appears that the material significantly reduced cell viability compared to the control at only the highest dose of 1% concentration, and this decrease increased in a time-dependent manner. It is noteworthy that the cell viability does not decrease from 0.1% dose and even increases statistically significantly compared to the control. This suggests that there is no cytotoxicity due to glutaraldehyde.
Hanks et al. [38] examined the cytotoxic effects of two different dentin bonding systems and their contents (Gluma/ glutaraldehyde, Scotchbond2/HEMA) on BALB/3T3 cells. Researchers have reported that glutaraldehyde was more toxic than HEMA and yet both binding systems showed similar cytotoxic effects. In our study, Gluma 2 Bond with glutaraldehyde content was used and it was observed that there was no significant decrease in vitality as the dose increased. Viability (at the highest dose only) decreased in a time-dependent manner [39,40].
No study on the cytotoxicity of Peak Universal Bond with chlorhexidine, which is one of the antibacterial effective adhesive systems we use in our study, has been found. In our study, it was seen that Peak Universal Bond significantly reduced cell viability and high cytotoxic effect for all incubation times compared to control at all doses tested (p<0.001***).
In the 24-hour incubation, we found that cell viability did not change significantly with low doses. In 4-and 72- hour incubation periods, only 0.01% and 0.1% doses were found to be different. There was no significant change in cell viability between either 0.001%/0.01% and 0.1%/1% dose groups.
Conclusion
According to the results of our study, the antibacterial components added to the adhesive systems had no adverse effect on biocompatibility. It has been observed that the highest adhesive dose causes the most toxic effect in both adhesive systems with antibacterial components and adhesive systems without antibacterial components, and this situation increases with the incubation period. However, further research is required before clinical use for Peak Universal Bond, the chlorhexidine-containing adhesive system, which has a high cytotoxic effect compared to other antibacterial adhesive systems.
Declaration of Interest
The authors have no conflicts of interest relevant to this article.
References
- Tyas MJ, Anusavice KJ, Frencken JE. Mount GJ. Minimal intervention dentistry: A Review. FDI Commission Project 1–97. Int Dent J 2000; 50: 1-12.
- Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. State of the art of self-etch adhesives. Dent Mater 2011; 27: 17-28.
- Bakır S, Bakır EP, Süsgün Yildirim Z. Biocompatibility of Dental Adhesives. Adv Dent& Oral Health 2017; 4: 555-643.
- Marshall SJ, Bayne SC, Baier R, Tomsia AP, Marshall GW. A review of adhesion science. Dent Mater 2010; 26: e11-e16.
- Moszner N, Salz U, Zimmermann J. Chemical aspects of self-etching enamel–dentin adhesives: A systematic review. Dent Mater 2005; 21: 895-910.
- Ratanasathien S, Wataha J, Hanks C, Dennison J. Cytotoxic interactive effects of dentin bonding components on mouse fibroblasts. J Dent Res 1995; 74: 1602-1606.
- Süsgün Yildirim Z, Bakır S, Bakır E, Foto E. Qualitative and Quantitative Evaluation of Cytotoxicity of Five Different One-Step Self-Etching Adhesives. Oral Health Prev Dent 2018; 16: 525-532.
- Gerzina T, Hume W. Effect of dentine on release of TEGDMA from resin composite in vitro. J oral Rehabil 1994; 21: 463-468.
- Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 2006; 27: 3995-4002.
- Imazato S, Ebi N, Tarumi H, Russell RR, Kaneko T, Ebisu S. Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials 1999; 20: 899-903.
- Koulaouzidou EA, Helvatjoglu-Antoniades M, Palaghias G, Karanika-Kouma A, Antoniades D. Cytotoxicity of dental adhesives in vitro. Eur J Dent 2009; 3: 3-9.
- Sofan E, Sofan A, Palaia G, Tenore G, Romeo U, Migliau G. Classification review of dental adhesive systems: from the IV generation to the universal type. Ann Stomatol 2017; 8: 1.
- Kenawy ER, Worley S, Broughton R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Bio macromolecules. 2007; 8: 1359-1384.
- Fardai O, Turnbull RS. A review of the literature on use of chlorhexidine in dentistry. J Am Dent Assoc 1986; 112: 863-869.
- Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin Diagn Lab Immunol 1999; 6: 437-439.
- Keskin Y, Kansu G. The Effect of Sterilization Methods on the Dimensional Stability of Rock Resins Polymerized by Different Methods. J Dental Sci 1999; 5: 92-98.
- Al-Dawood A, Wennberg A. Biocompatibility of dentin bonding agents. Endod Dent Traumatol 1993; 9: 1-7.
- Schmalz G. Concepts in biocompatibility testing of dental restorative materials. Clin Oral Investig 1997; 1: 154-162.
- Susgun Yildirim Z, Bakir EP, Bakir S, Aydin MS, Biocompatibility and evaluation methods in dentistry. Selcuk Dent J 2017; 4: 162-169.
- Wataha JC. Principles of biocompatibility for dental practioners. J Prosth Dent 2001; 86: 203-209.
- Yalcin M, Kenar H, Dayı B, Şişman R, Karaoz E. The Effect of Light Curing Units on Proliferation and Senescence of Human Dental Pulp Mesenchymal Stem Cells. IJDSCR 2016; 4: 10-16.
- Cao T, Saw TY, Heng BC, Liu H, Yap AUJ, Ng ML. Comparison of different test models for the assesment of cytotoxicity of composite resins. J Appl Toxicol 2005; 25: 101-108.
- Sletten GB, Dahl JE. Cytotoxic effects of extracts of compomers. Acta Odontol Scand 1999; 57: 316-322.
- Wataha JC. Principles of biocompatibility for dental practioners. J Prosth Dent 2001; 86: 203-209.
- ISO 7405. Dentistry - Preclinical evaluation of biocompatibility of medical devices used in dentistry-Test methods for dental materials. International Standards Organization 1996.
- Issa Y, Watts D, Brunton P, Waters C, Duxbury A. Resin composite monomers alter MTT and LDH activity of human gingival fibroblasts in vitro. Dent Mater 2004; 20: 12-20.
- Paull KD, Shoemaker RH, Boyd MR, Parsons JL, Risbood PA, Barbera WA. The synthesis of XTT: A new tetrazolium reagent that is bioreducible to a water‐soluble formazan. J Heterocycl Chem 1988; 25: 911-914.
- Li JL, Wang WM, Ge JY, Ji J, Wang TC. A comparative study ofthe cytotoxicity of five dental bonding agents to human period ontalligament cells. Shanghai J Stomato 22: 628-633.
- Chen RS, Liu CC, Tseng WY, Jeng JH, Lin CP. Cytotoxicity of three dentin bonding agents on human dental pulp cells. J Dent 2003; 31: 223-229.
- Cal E, Guneri P, Atay A, Cetintas VB. Cytotoxicity of dentin bonding agents. Gen Dent 2014; 62: 11-14.
- Hanks C, Strawn S, Watahai J, Craig R. Cytotoxic effects of resin components on cultured mammalian fibroblasts. J Dent Res 1991; 70: 1450-1455.
- Katz S. In-vitro root surface caries studies. J Oral Med 1987; 42: 40-48.
- Imazato S, Kaneko T, Takahashi Y, Noiri Y, Ebisu S. In vivo antibacterial effects of dentin primer incorporating MDPB. Oper Dent 2004; 29: 369-375.
- Ozer F, Karakaya Ş, Unlu N, Erganiş O, Kav K, Imazato S. Comparison of antibacterial activity of two dentin bonding systems using agar well technique and tooth cavity model. J Dent 2003; 31: 111-116.
- Grobler SR, Oliver A, Moodley D, Van Wyk Kotze TJ. Cytotoxicity of recent dentin bonding agents on mouse fibroblast cells. Quintessence Int 2008; 39: 511-516.
- Wiegand A, Caspar C, Becker K, Werner C, Attin T. In vitro cytotoxicity of different self-etching dental adhesive systems. Schweiz Monatsschr Zahnmed. 2006; 116: 614-621
- Imazato S, Kuramoto A, Takahashi Y, Ebisu S, Peters MC. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dent Mater 2006; 22: 527-532.
- Hanks C, Wataha J, Parsell R, Strawn S. Delineation of cytotoxic concentrations of two dentin bonding agents in vitro. J Endod 1992; 18: 589-596
- Feigal RJ, Yesilsoy C, Messer HH, Nelson J. Differential sensitivity of normal human pulp and transformed mouse fibroblasts to cytotoxic challenge. Arch Oral Biol 1985; 30: 609-613.
- Mali P, Deshpande S, Singh A. Microleakage of restorative materials: an in vitro study. J Indian Soc Pedod Prev Dent 2006; 24: 15.