- Biomedical Research (2014) Volume 25, Issue 3
Evaluation of anabolic hormone status in patients with COPD during stable and acute exacerbation state.
Gupta M1*, Vardey SK1, Sinha M4, Joshi N2, Dixit R3 and Gupta R51Department of Biochemistry, SMS Medical College and Hospital, Jaipur, Rajasthan. India
2Institute of Respiratory Diseases, SMS Medical College and Hospital, Jaipur, Rajasthan. India
3Department of Respiratory Medicine, JLN Medical College, Ajmer, Rajasthan. India
4Mahatma Gandhi Institute of Medical Sciences, Jaipur, Rajasthan. India
5ESI Hospital, Jaipur, Rajasthan. India
Accepted date: April 14 2014
Abstract
Adequate levels of anabolic hormones are required for normal muscle growth and development. Decreased levels of anabolic hormone are described in chronic obstructive pulmonary disease (COPD), leading to important clinical consequences. The aim of this study was to evaluate the circulating levels of anabolic hormones in stable and acute exacerbation cases of COPD compared to age-matched control subjects. A total of 146 (70 stable and 76 in acute exacerbation) male COPD patients and 79 age-matched control subjects were admitted in our study. Pulmonary function test (PFT), arterial blood gas (ABG) analysis and circulating levels of anabolic hormones, such as growth hormone (GH), insulin-like growth factor- 1 ((IGF-1), dehydroepiandrosterone sulphate (DHEAS) and testosterone were measured and compared. Serum IGF-1, DHEAS and testosterone levels were found to be significantly lower in stable and acute exacerbation COPD (AECOPD) patients compared to healthy controls (p<0.001). In both the COPD groups, GH was also found to be significantly (p<0.000) lower in comparison with the controls. However, there was no significant difference in mean serum GH values between stable and AECOPD groups. In COPD patients FEV1 and FEV1/FVC (%), pO2, SO2 (%) were significantly correlated with IGF-1 and testosterone levels. PCO2 and HCO3 levels were found to be inversely correlated with IGF-1 and testosterone levels (p<0.05). Disturbance in anabolic hormones in COPD patients may have potential effects in the development of peripheral muscle weakness. Chronic hypoxemia or hypercapnia could conceivably damage the muscles because of their correlation with changes in the status of anabolic hormones. The decrease in anabolic hormones may influence the clinical picture and perhaps the survival of the patients with COPD.
Keywords
COPD, Growth hormone, IGF-1, DHEAS, testosterone, acute exacerbation, pulmonary function test, arterial blood gases
Introduction
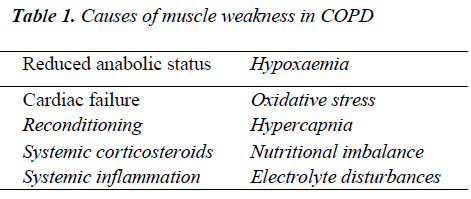
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and mortality throughout the world. In a recent study, WHO estimated it to be the third leading cause of death by 2030 [1]. COPD is characterized by significant chronic inflammation in the pulmonary compartments, also associated with systemic alterations in biochemistry and organ function [2,3]. Several factors such as hypoxemia, hypercapnia, exacerbation, drugs, malnutrition may lead to endocrinological changes in COPD [4]. Disturbances in the anabolic hormone system may also impair the anabolic responses needed for skeletal muscle performance. It is postulated that skeletal muscle dysfunction in COPD arises from two complex, interrelated events: muscle mass depletion caused by mitochondrial abnormalities and loss of contractile proteins, and muscle dysfunction or malfunction of the remaining muscle [5]. The pathophysiological mechanisms underlying skeletal muscle dysfunction have not been fully elucidated, however, while anabolic hormonal imbalances have been shown to be involved, a host of other factors has also been implicated (Table 1).
We therefore, aimed to correlate the levels of anabolic hormones with changes in arterial blood gas and pulmonary function test among stable and exacerbated COPD patients compared to age-matched healthy controls. To the best of author’s knowledge such kind of study has not been conducted in Indian patients previously.
Materials and Methods
Subjects
The study group comprised of 146 male COPD patients and 79 age-matched control subjects. The patients were subdivided into stable and acute exacerbation groups. COPD patients were diagnosed according to the Global Initiative for Chronic Obstructive Lung Disease Criteria [6] by the respiratory physician. Seventy of them were clinically stable patients for at least 3 months and 76 had clinical signs of COPD exacerbation. Stable COPD patients had been receiving inhaled bronchodilator therapy in the form of long-acting β2-agonists and/or anticholinergic agents. Severe/very severe COPD patients were on inhaled corticosteroids. Antibiotics and systemic steroids (methylprednisolone 40 mg/day) were added to therapy only in exacerbation period for 10-14 days after the blood samples were taken. No patients were receiving long-term oxygen therapy. Patients with concomitant diseases such as malignancy, infection other than respiratory tract infections, active pulmonary tuberculosis, cardiac failure, severe endocrine disorder, hepatic or renal diseases, systemic autoimmune disorders and patients, those were on drugs which may interfere with serum hormone levels were excluded from this study. From a total 146 patients, 45 were ex-smokers, 87 were current smokers and 14 were nonsmokers. Control subjects were non-smokers and not on any medication. The institutional ethics committee approved the study and written consent was obtained in each case.
Study design
Clinical history was obtained and physical examination performed on the patients who attended the institute of respiratory diseases, SMS Medical College and Hospital, Jaipur. After an overnight fast, height and nude weight were recorded in all subjects and body mass index (BMI) was calculated. Arterial blood gas measurements were taken on the same day along with PFT. PFT was performed using Spiro-Analyzer HELIOS 400. Arterial blood samples were drawn while the subjects were breathing room air and analyzed with a blood gas analyzer immediately (STAT PRO PHOX Plus L, Nova Biomedical). Serum hormone levels were measured in 10 ml venous blood sample. hGH and testosterone were measured once in stable COPD patients and controls, also measured on the same day in acute exacerbation COPD patients during hospitalization. For DHEAS and IGF-1, serum was stored at -40ºC until analyzed. hGH and testosterone were estimated by using the commercial kits (Immulite, Siemens Healthcare Diagnostics, U.K.) on Immulite 2000. DHEAS and IGF-1 were measured by solid phase enzyme linked immunosorbent assay based (ELISA) kits supplied by DiaMetra S.r.l, Italy and DRG Diagnostics, Germany, respectively on ELISA reader.
Statistical Analysis
Results were expressed as the mean ± SD. Continuous variables were summarized as mean and Std. Deviation, while categorical variable and data were on nominal scale as proportions (%). Parametric tests like unpaired ‘t’ test , one way ANOVA analysis with Post hoc-Tukey’s HSD and Pearson’s correlation coefficient were used for analysis of continuous variables. A p value < 0.05 was considered to be statistically significant.
Results
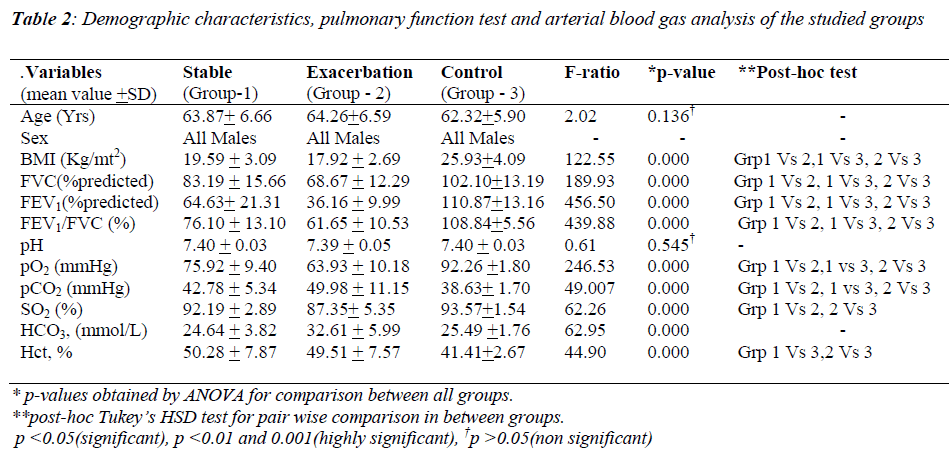
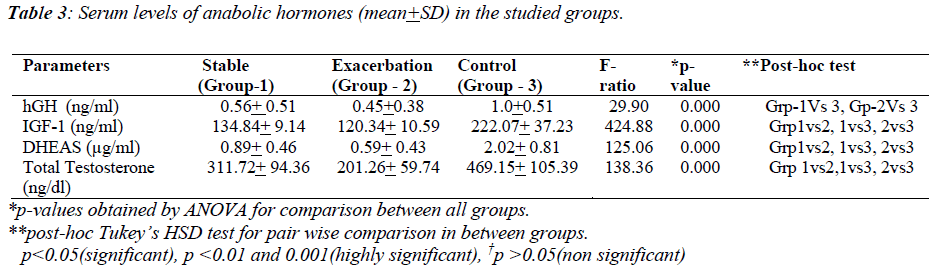
The characteristics of male COPD patients and healthy controls are shown in Table-2. Subjects of studied groups were age-matched. However, between the groups 1 (stable COPD), 2 (AECOPD) and 3 (controls), BMI was found to be significantly different All the AECOPD patients were suffering from severe to very severe airway obstruction with an FEV1 36.16 ± 9.99 of percentage predicted as compared to stable COPD patients (64.63±21.31). All COPD patients of group 1 and group 2 were hypoxemic with a significant difference in pO2 as compared to healthy controls (92.26±1.80vs.75.92±9.40 and 63.93±10.18), respectively. Group 2 patients were hypercapnics as compared to group 1 and group 3 (49.98±11.15 vs. 42.78±5.34 and 38.63±1.70), respectively (p< 0.000). Table-3 shows the comparison between stable, AECOPD and control groups regarding hGH, IGF- 1, testosterone and DHEAS. In stable and AECOPD groups, hGH was found to be significantly (p<0.000) lower in comparison with the control group. No significant difference in mean serum GH levels recorded between stable and AECOPD groups. A significant difference was, however, found between three groups for serum IGF-1. IGF-1 was significantly (p<0.000) lower in the AE group of COPD in comparison with the stable COPD and control group. IGF-1 was also significantly lower in stable COPD group in comparison with the control group. DHEAS and testosterone levels were significantly (p<0.000) lower in AECOPD group in comparison with stable COPD and control group. A significantly (p<0.000) lower value was also obtained in stable COPD group in comparison with the control group.
Correlations
A significant positive correlation was found between testosterone and BMI in acute AECOPD patients (r=0. 28; p=0. 023). We also found positive correlation of IGF-1 and testosterone with FVC%, FEV1% and FEV1/FVC% and also a positive correlation of DHEA-S with FEV1/FVC% (r=0. 342; p=0. 004) in stable COPD patients. A positive correlation of IGF-1 with pO2 and % SO2 and a negative correlation of hGH with pCO2 were also obtained. A positive correlation of FEV1% with IGF- 1 and testosterone, also a positive correlation of FEV1/FVC% with IGF-1, DHEAS and testosterone were recorded in AECOPD patients. A negative correlation was found between IGF-1 and HCO3 and a positive correlation of GH with Hct (%) and DHEAS with pO2 were also observed during acute exacerbation phase in COPD patients.
Discussion
As adequate levels of anabolic hormones are required for normal muscle growth and development [7], it has been reported that substantially reduced levels of growthpromoting factors, such as IGF-1 and testosterone may contribute to muscle dysfunction in COPD [8]. Thus, we investigated the anabolic hormone levels among stable and AECOPD patients. The control of muscle mass is complex and includes the action of inflammatory cytokines, mechanical load on the muscles and anabolic hormones. There are four main anabolic axes: somatotropic, gonadal, adrenal and insulin [9-12]. Although the GH/IGF-I axis is often considered a major regulator of muscle mass, there is accumulating evidence that IGF-I also act independently of GH [13].
Similar to our findings for GH, Xu et al. [14] found changes of ghrelin, growth hormone, growth hormone releasing hormone and their clinical significances in patients with COPD. They noticed lower plasma GH in the underweight patients compared to the patients with normal body weight. Debigare et al. [15] conversely, demonstrated an increase in circulating GH and IGF-1 in patients with COPD and suggested that physiological stress like chronic hypoxia and bronchoconstriction could possibly resulted in an increase in growth hormone secretion [16].
IGF-1 has been implicated in several important functions such as cell differentiation, growth and maintenance of skeletal muscle. Circulating IGF-1 level has been used as a marker of growth hormone action because IGF-1 has a longer half-life than GH, also integrates the pulsatile release of GH [3]. Similar to our findings, Prokopis et al. [17] reported low IGF-1 levels in COPD patients on day 1 of the exacerbation with respect to healthy controls. The levels of IGF-1 found to be significantly increased from day 1 to day 15 of exacerbation (p < 0.001). The authors concluded that GH mediates its major metabolic effects predominantly through IGF-1. In our study, patients with COPD showed significantly lower IGF-1 on exacerbation and also on stable stage compared to healthy controls. Our finding of low IGF-1 levels is compatible with other studies. Casaburi et al. [8] have also reported low levels of IGF-1 in COPD patients. Spruit et al. [18] found that IGF-1 levels tended to be lower in patients with COPD exacerbation than in healthy subjects. Funda et al. [19], however, reported decreased circulating levels of IGF-1 in stable COPD patients compared to healthy controls, which is related to the severity of disease.
We are aware that the bioavailability and the effects of IGF-1 are influenced by IGF-1 binding proteins (IGFBP). Therefore, systemic inflammation causes an increase in circulating levels of these proteins may decrease the levels of free IGF-1. Cytokines increase IGFBP-1 and IGFBP-4, which result in a decreased free IGF-1 fraction. IL-6 suppresses IGF-1 via increased production of its binding protein [20]. Previous studies indicated that intravenous or oral corticosteroids may affect circulating IGF- 1 levels.
This effect is thought to be associated with dosage and time course, but it remains controversial as to whether circulating IGF-1 levels are increased or decreased in response to corticosteroids. Schuetz et al. [21] reported that a chronic systemic steroid therapy may influence circulating IGF-1 levels by suppressing the GH–IGF axis, however, corticosteroids may allow short-term recovery of the levels of circulating IGF-1 by abating inflammation. Patients receiving intravenous or oral corticosteroid therapy were excluded from the present study in order to eliminate these possible interfering effects. Shin et al. [22] found that age and gender may affect serum IGF-1. Since these possibility had been avoided in the present study, therefore the reduction in circulating IGF-1 levels in the patients with AECOPD appear to be related to COPD exacerbation. Furthermore, the increase in IGF-1 levels in CSCOPD patients compared with AECOPD patients suggest that systemic inflammation and endocrine disorder may be ameliorated during this clinically stable stage. Further studies are needed to elucidate the reliability and reference range of IGF-1 levels during COPD exacerbation.
Our results are also in agreement with Spruit et al. [18] who reported that lower levels of serum IGF-1 are correlated with lower FEV1 values in COPD patients, which might be consistent with the impression that the GH/IGF-1 axis is suppressed by severe chronic disease [16].
Similar to our results, Ursavas et al. [23] reported a correlation between low IGF-1 with low PaO2, since acute hypoxemia decreases the rate of IGF-1 synthesis, increases clearance rate of IGF-1, or increased plasma concentration of IGFBP-1, which decreases the free IGF-1 fraction. Chronic hypoxemia also exerts a direct effect on transcription of IGF-mRNA, similar to its effect on transcription of other proteins [24].
Akbas et al. [26] suggested that decreased levels of testosterone in COPD patients could result from a primary testicular atrophy in COPD. Our findings also tallied with Karadag et al. [27] who have speculated that chronic hypoxia, severity of the disease, smoking, corticosteroid therapy and chronic (inflammatory) illness contribute to low testosterone levels. Gosney [28] found smaller testis volume and Leydig-cell atrophy in COPD patients, and suggested that this atrophy may be a consequence of hypoxic inhibition of the pituitary synthesis or release of LH. In contrary, Svartberg et al. [29] reported that men having chronic bronchitis or emphysema didn’t have reduction in testosterone levels ,thus they speculate that it is not the disease per se that affects the hormone levels but the actual level of pulmonary function. Also, they hypothesize that the gradual decline inFEV1% predicted is followed by a decline in testosterone and this may explain the decrease in serum testosterone levels with increasing severity of COPD.
Debigare et al. [15] reported lower DHEAS along with decreased muscle mass in patients with COPD. DHEAS is the sulphated metabolite of DHEA, produced by adrenal cortex and is the most abundant steroid present in the blood. DHEAS may act directly at the tissue level or after its conversion to androstenedione or androstenediol, and finally to testosterone [15]. Testosterone increases net protein synthesis within the muscle using the intracellular amino acid pool [39]. In aging men and women, DHEAS may contribute to anabolism indirectly by increasing serum IGF-1[40], although the discovery of specific binding sites for DHEAS on skeletal muscle cells supports a direct action of DHEAS on these cells [15]. In our patients, the possible mechanism of low blood concentration of testosterone remains enigmatic. Karadag et al. [27] found decreased DHEAS in patients with COPD, which is decreased even further during acute exacerbations. This reduced concentration of DHEAS is thought to create an imbalance between protein synthesis and degradation favouring catabolism over anabolism, which is possibly rested in peripheral muscle wasting.
As anabolic hormonal imbalance caused muscle dysfunction in COPD, hormone supplementation seems to be an attractive method to reverse muscle dysfunction. Anabolic hormone, such as testosterone, supplementation has been suggested to treat muscle wasting and dysfunction during COPD [30,31]. Moreover a combination of strength training and testosterone supplementation appeared to give additive effects on lean body mass and strength in patients with COPD [30]. However, the long-term (side and beneficial) effects of hormone replacement remain to be established. It has been reported that IGF-1 is only related to muscle strength when IL-6 levels are low [32] suggesting that the effectiveness of testosterone replacement (which probably acts in part through an effect on IGF-1) may be attenuated in patients with high levels of systemic inflammation. It has also been reported that muscle weakness has important consequences, including exercise limitation, [33-35] reduced QoL, enhanced utilization of health care resources [36] and reduced survival [37,38].
We considered few limitations in this study. First, the serum levels of anabolic factors were evaluated on only one occasion in the morning. Although daily fluctuations in the blood levels of these factors may occur, serial sampling could have provided a better overview of the hormonal status in our COPD patients. Second, the females patients could not be evaluated due to the reason mentioned previously & the fewer number of COPD patients visiting our centre.
In conclusion, this study reconfirm that COPD patients have a low anabolic hormone status, which can be caused by a combination of factors and this decrease is more marked during exacerbation period when hypoxaemia is more significant. Anabolic hormones are required for normal muscle growth and development; therefore its deficiency may lead to dysfunction of the peripheral musculature of patients with COPD. Further work is necessary to elucidate the exact mechanism that results in anabolic hormone imbalance-related muscle weakness in COPD.
References
- World Health Organization. Burden of COPD. Available from: http://www.who.int/respiratory/copd/burden/en/
- Vernooy JH, Kucukaycan M, Jacobs JA, et al. Local and systemic inflammation in patients with chronic obstructive pulmonary disease: soluble tumor necrosis factor receptors increase in sputum. Am J Respir Crit Care Med 2002; 166: 1218-1224.
- Wouters EF, Creutzberg EC, Schols AM. Systemic effects in COPD. Chest 2002; 121(Suppl.): 127s-30s.
- Karadag F, Ozcan H, Karul AB, et al. Correlates of non-thyroidal illness syndrome in chronic obstructive pulmonary disease. Respir Med 2007; 101: 1439-1446.
- Agusti AGN, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 2003; 21: 347-360.
- Roberto R, Antonio A, Jean B, et al. Pocket Guide to COPD diagnosis, management and prevention: Global Initiative for Chronic Obstructive Lung Disease (GOLD) updated 2010.
- Casaburi R. Skeletal muscle Function in COPD. Chest 2000; 117(5): 267s-271s
- Casaburi R, Goren S, Bhasin S. Substantial prevalence of low anabolic hormone levels in COPD patients undergoing rehabilitation. Am J Respir Crit Care Med 1996; 153: A128.
- Creutzberg EC, Schols AMWJ, Bothmer-Quaedvlieg FCM, Wouters EFM. Prevalence of elevated resting energy expenditure in patients with chronic obstructive pulmonary disease in relation to body composition and lung function. Eur J Clin Nutr 1998; 52: 396-401.
- Wagner PD. Skeletal muscles in chronic obstructive pulmonary disease: deconditioning, or myopathy? Respirology 2006; 11: 681-686.
- Maggio M, Lauretani F, Ceda GP, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (In CHIANTI) study. Arch Intern Med 2007; 167: 2249-2254.
- Bolster DR, Jefferson LS, Kimball SR. Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc Nutr Soc 2004; 63: 351-356.
- Crul T, Spruit MA, Gayan-Ramirez G. Markers of inflammation and disuse in vastus lateralis of chronic obstructive pulmonary disease patients. Eur J Clin Invest 2007; 37: 897-904.
- Xu ZS, Bao ZY, Wang ZY, Yang GJ, Zhu DF, Zhang L, Tan RM. The changes of ghrelin, growth hormone, growth hormone releasing hormone and their clinical significances in patients with chronic obstructive pulmonary disease. Zhonghua Nei Ke Za Zhi 2012; 51(7): 536-539.
- Debigare R, Marquis K, Cote CH, et al. Catabolic/anabolic balance and muscle wasting in patients with COPD. Chest 2003; 124: 83-89.
- Scalvini S, Volterrani M, Vitacca M, et al. Plasma hormone levels and haemodynamics in patients with chronic obstructive lung disease. Monaldi Arch Chest Dis 1996; 51: 380-386.
- Prokopis K, Ageliki K, et al. Plasma leptin and insulinlike growth factor I levels during acute exacerbations of chronic obstructive pulmonary disease.BMC Pulm Med 2009; 9: 11.
- Spruit MA, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax 2003; 58: 752-756.
- Funda C, Ercüment EGE, et al. Evaluation of thyroid hormone levels and somatomedin-C (IGF-1) in patients with chronic obstructive pulmonary disease (COPD). Tüberküloz ve Toraks Dergisi 2009; 57(4): 369-375.
- Banbassat CA, Lazarus DD, Cichy SB. Interleukin-1 alpha (IL-1alpha) and tumor necrosis factor alpha (TNF-a) regulate insulin-like growth factor binding protein-1(IGFBP-1) levels and mRNA abundance in vivo and in vitro. Horm Metab Res 1999; 31: 209-215.
- Schuetz P, Christ-Crain M, Schild U, et al. Effect of a 14-day course of systemic corticosteroids on the hypothalamic-pituitaryadrenal- axis in patients with acute exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med 2008; 8: 1.
- Shin SY, Lee JR, Noh GW, et al: Analysis of serum levels of anti-Mullerian hormone, inhibin B, insulinlike growth factor-I, insulinlike growth factor binding protein-3, and follicle-stimulating hormone with respect to age and menopausal status. J Korean Med Sci 2008; 23: 104-110.
- Ursavas, M. Karadag, Y.O. Ilcol. Low level of IGF-1 in obesity may be related to obstructive sleep apnea syndrome. Lung 2007; 185: 309-314.
- Bernstein D, Doshi R, Huang S, et al. Transcriptional regulation of left ventricular B-adrenergic receptors during chronic hypoxia. Pediatr Res 1991; 29: 15A.
- Spratt DI. Altered steroidogenesis in critical illness: is treatment with anabolic steroids indicated? Best Pract Clin Endocrinol Metab 2001; 15: 479-494.
- Turkay A, Karakurt S, et al. The endocrinologic changes in critically ill chronic obstructive pulmonary disease patients. J COPD 2010; 7: 240-247.
- Karadag F, Ozcan H, Karul AB, et al. Sex hormone alterations and systemic inflammation in chronic obstructive pulmonary disease. Int J Clin Pract 2007; 63: 275-281.
- Gosney JR: Atrophy of leydig cells in the testes of men with long standing chronic bronchitis and emphysema. Thorax 1987; 42: 615-619.
- Svartberg J, Schirmer H, and Medbo A, et al. Reduced pulmonary function is associated with lower levels of endogenous total and free testosterone. European Journal of Epidemiology 2007; 22: 107-112.
- Casaburi R, Bhasin S, Cosentino L, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 170: 870-878.
- Hansen MJ, Gualano RC, Bozinovski S, et al. Therapeutic prospects to treat skeletal muscle wasting in COPD (chronic obstructive lung disease). Pharmacol Ther 2006; 109: 162-172.
- Barbieri M, Ferrucci L, Ragno E, et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab 2003; 284:E481-E487.
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996; 153: 976-980.
- Hamilton AL, Killian KJ, Summers E, Jones NL. Muscle strength, symptom intensity, and exercise capacity in Systemic effects of COPD S9 patients with cardiorespiratory disorders. Am J Respir Crit Care Med 1995; 152: 2021-2031.
- Saey D, Debigare R, Leblanc P, et al. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 168: 425-430.
- Decramer M, Gosselink R, Troosters T, Verschueren M, Evers G. Muscle weakness is related to utilization of health care resources in COPD patients. Eur Respir J 1997; 10: 417-423.
- Marquis K, Debigare R, Lacasse Y, et al. Midthigh muscle crosssectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166: 809-813.
- Marc D, Fernando De B, Adriana DP, Stefano M. Systemic effects of COPD. Resp Med 2005; 99: S3-S10.
- Ferrando AA, Tipton KD, Doyle D, et al. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol 1998; 275: E864-E871.
- Morales AJ, Haubrich RH, Hwang JY, et al. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in ageadvanced men and women. Clin Endocrinol (Oxf) 1998; 49: 421-432.