- Biomedical Research (2014) Volume 25, Issue 3
Evaluating the anti-angiogenic properties of Iloprost and Dipyridamole in the chick embryo chorioallantoic membrane model
Orkut Guclu*, Oguz Karahan, Suleyman Yazici, Ahmet Caliskan, Sinan Demirtas, Celal Yavuz, Abdurrahman Muratoglu, Binali MavitasMedical School of Dicle University, Department of Cardiovascular Surgery, Diyarbakir, Turkey
- *Corresponding Author:
- Orkut GUCLU
Medical School of Dicle University
Department of Cardiovascular Surgery
Diyarbakir/TURKEY
Accepted date: April 04 2014
Abstract
Dipyridamole is an antithrombotic agent that is widely used in the treatment of many vascular disorders. Also, the prostacyclin analogue iloprost has been utilized to salvage limbs in patients with severe limb ischemia. In this study we investigated whether dipyridamole and iloprost have anti-angiogenic properties and their anti-angiogenic properties were compared to bevacizumab, a known inhibitor of angiogenesis, using the in vivo chick chorioallantoic membrane animal model. Agar pellets were prepared with three different drug concentrations at 10-6 M, 10-5 M, and 10-4 M. For each drug concentration twenty fertilized eggs were used. The entire experiment was performed in duplicate. Blood vessel density and loss were examined and scored under a stereoscopic microscope. For the 10-4 M, 10-5 M and 10-6 M concentrations, the anti-angiogenic scores of iloprost were 0.2, 0.1 and 0.05, respectively. In the same order, the anti-angiogenic scores for dipyridamole were 0.2, 0.3 and 0.8. The antiangiogenic scores for bevacizumab were significantly higher than dipyridamole and iloprost over all concentrations (p<0.05). There were no significant differences found between the anti-angiogenic scores for iloprost and dipyridamole for all concentrations (p>0.05). Iloprost demonstrated no anti-angiogenic properties in the chorioallantoic membrane animal model, while dipyridamole did exhibit very weak anti-angiogenic activity only at very high doses of 10-4 M. These results reveal that both agents can be prescribed safely for the treatment of medical conditions that require angiogenesis to facilitate healing.
Keywords
Iloprost, dipyridamole, bevacizumab, angiogenesis, chorioallantoic membrane model
Introduction
Neo-angiogenesis is one of the body’s natural responses to counteract tissue ischemia in order to sustain cellular metabolism [1]. Research is actively being performed to determine how to promote neo-angiogenesis in medical illnesses that are characterized by low-perfusion states, and advances in therapeutic angiogenesis are progressing rapidly [1,2]. Iloprost is a prostacyclin (Prostaglandin I2; PGI2) analogue that has become routinely utilized in treating occlusive vascular diseases. Besides iloprost, dipyridamole is also used to treat vascular diseases as it inhibits phosphodiesterase and increases intracellular levels of cyclic adenosine monophosphate and cyclic guanosine monophosphate as well as extracellular concentrations of adenosine [3]. Furthermore, both of these agents promote the inhibition of platelet aggregation and vasodilatation [4].
Iloprost is administered to treat various vascular diseases including intermittent claudication, ischemic leg ulcers, pulmonary hypertension, and peripheral vascular disorders [4,5]. Dipyridamole is traditionally prescribed as an anti-platelet agent and is used for the treatment of many cardiovascular diseases such as ischemic cardiomyopathy, pulmonary hypertension, and stroke [6]. Iloprost and dipyridamole may also be used in the treatment of inflammatory disorders as both inhibit inflammation by antagonizing pro-inflammatory cytokines [7]. Several studies have suggested that these drugs might play a role in modulating cytokines that promote angiogenesis, but the effects of these drugs on angiogenesis still remains unclear [2].
Chick embryo chorioallantoic membrane (CAM), rabbit cornea, and rodent mesenteric animal models are the most commonly used in vivo models of angiogenesis. The CAM model is often used, as it is a low-cost and overall simpler model to work with, which facilitates experiment reproducibility [8]. Bevacizumab antagonizes angiogenesis and is a known inhibitor of vascular endothelial growth factor (VEGF) [9]. In this study, we aimed to determine whether iloprost and dipyridamole inhibits angiogenesis in comparison to bevacizumab in the CAM animal model.
Materials and Methods
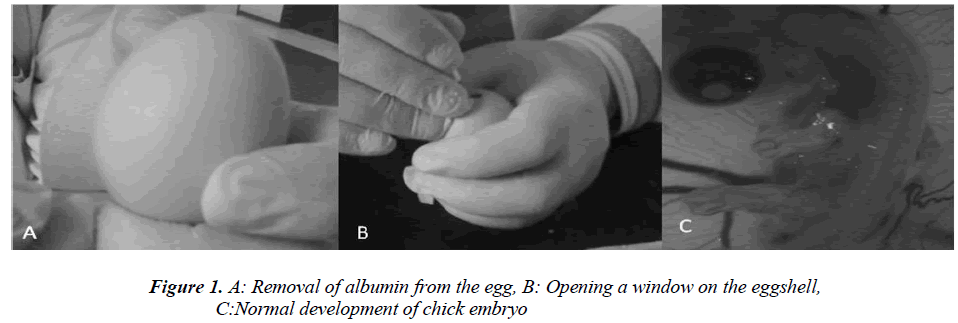
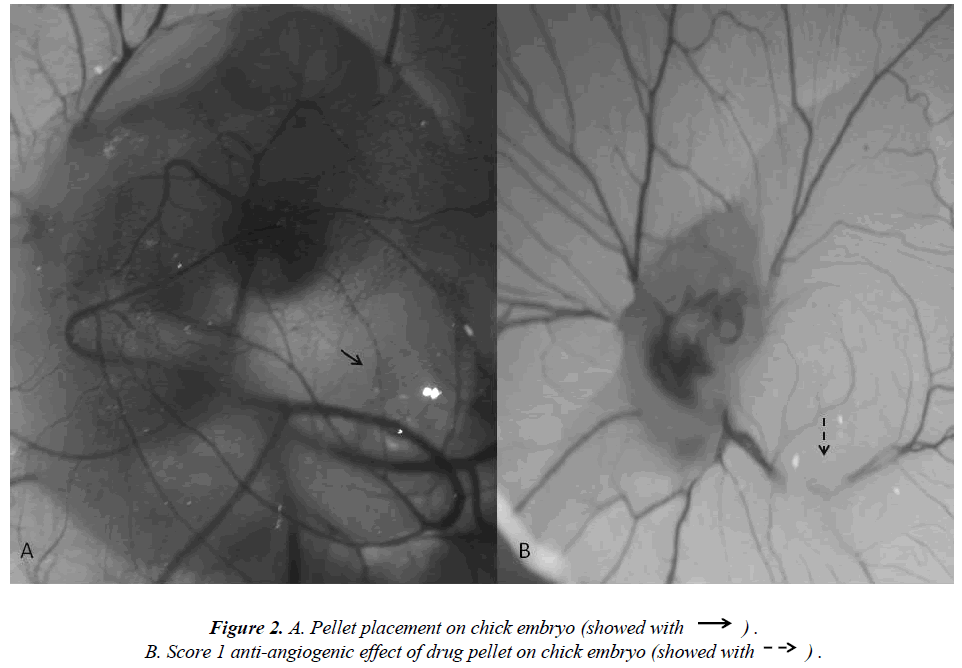
Fertile hen eggs were obtained locally and were incubated at 37.5 °C in 80% relative humidity. The eggs were rotated twice daily and on Day 5 of incubation the egg surface and all instruments used to handle the eggs were disinfected with 70% isopropyl alcohol. Then 5 ml of albumin was injected through the eggshell (Figure 1A) and a 1 cm by 1.5 cm window was carved (Figure 1B) on the opposite face of the eggshell so as to view the CAM. Normal CAM development was determined for each egg (Figure 1C) and malformed or dead embryos were excluded from the study. The carved eggshell windows were closed with a sterile drape and incubation continued without rotation until Day 8 when the CAM was expected to reach 2 cm in diameter. On Day 8 the sterile drape was removed and agar pellets impregnated with either iloprost, dipyridamole, or bevacizumab were placed on the CAM of each egg (Figure 1C). The drapes were replaced and the eggs were allowed to incubate for 24 hours. The level of angiogenesis around the pellet was determined after the incubation period. Twenty eggs were used for each drug tested, and pellets containing only agar comprised the negative control group. Degenerated eggs were changed with healthy ones. This experiment was performed in duplicate. Pellets that caused an inflammatory response resulting in embryo toxicity were excluded from the study. The study protocol was reviewed and approved by the Dicle University Animal Ethics Committee.
Pellet Preparation
Iloprost (Iloprost®, Schering AG, Berlin, Germany) was commercially available in a soluble infusion and dipyridamole (Persantin®, Boehringer Ingelheim, Berkshire, England) was packaged in a 10 mg/2 mL ampule. Agarose (Merck, Darmstadt, Germany) was added to distilled water to make a 2.5% weight of agarose to the total volume of solution mixture. This solution was then sterilized in the autoclave at 121°C under one atmosphere of pressure and then cooled in a sterile container to 37 °C. After cooling the drug was added into the mixture. Three different drug concentrations were prepared for each condition at 10-6, 10-5 and 10-4 M. Exactly 1 mL of combined agar and drug solution was prepared such that the solution was initially concentrated at 10 IU and then diluted to 1 IU/10 µl by serial dilutions with agarose solution. Almost eighty pellets were created for each drug concentration. Then a 10 µl drop of the agardrug solution at the desired concentration was pipetted into previously sterilized cylindrical stainless steel rods of 5 mm diameter to form circular pellets. The pellets were allowed to further solidify at room temperature under sterile conditions.
Angiogenesis Scorin
The extent of angiogenesis antagonism observed in the CAM in response to the drug-impregnated pellet was scored according to a method that was previously described using a stereoscopic microscope [10-12]. The scoring system specifically accounts for changes in capillary density around the pellet so to evaluate the degree of inhibition that an agent has on angiogenesis (Figure 2A). Specifically, a score of 0 indicated an absence of any verifiable anti-angiogenic effect and so was comparable to the capillary distribution of a normal embryo. A score 0.5 represented a very weak anti-angiogenic response and was characterized as having a reduced capillary density over an area equal to the pellet diameter with no capillary- free areas. A score of 1 represented a moderate effect as evidenced by a small capillary-free area or an area of significantly decreased capillary density less than twice the pellet size (Figure 2B). A score of 2 was given if a strong anti-angiogenic response was observed with a capillary-free area around the pellet that was equal to or greater than double the size of the pellet. The equation used for the determination of the average score is below:

According to this scoring system, a score of 0.5 specifically translated to no anti-angiogenic effect, whereas a score of 1 and above indicated some degree of antagonism of angiogenesis.
Statistical Analysis
Angiogenesis inhibition scores were compared with the Kruskal-Wallis ANOVA test and the Mann-Whitney U-test. A p-value less than 0.05 was considered statistically significant.
Results
Drug-free pellets containing a 10 µl volume of agarose solution were used as negative controls and did not induce any significant anti-angiogenic effect as their average anti-angiogenic score was 0.2. Pellets impregnated with bevacizumab were used as positive controls and on average had an anti-angiogenic score of 0.95 at the 10-6 M concentration. The anti-angiogenic properties of each drug was evaluated separately and then compared.
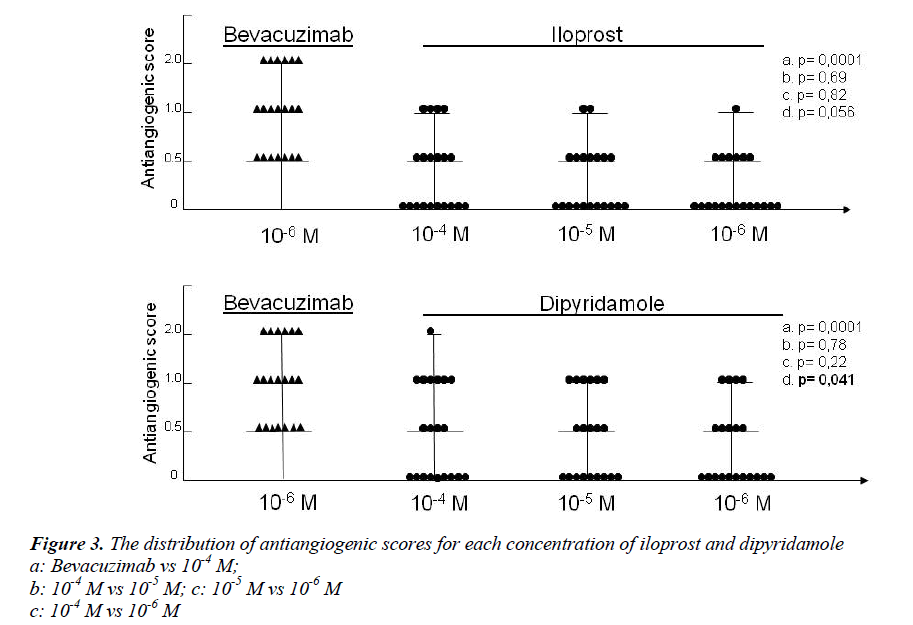
Figure 3 contains the anti-angiogenic scores obtained for iloprost, dipyridamole and bevacizumab. At 10-4 M, 10-5 M and 10-6 M concentrations, the mean anti-angiogenic scores of iloprost were 0.2, 0.1 and 0.05, respectively. In the same order, the average anti-angiogenic scores observed for dipyridamole were 0.8, 0.3 and 0.2. The antiangiogenic scores for bevacizumab were significantly higher than those observed for iloprost and dipyridamole over all concentrations (p<0.05). The anti-angiogenic score of dipyridamole was higher than iloprost in all concentrations. Only dipyridamole showed marked antiangiogenic tendency compared to iloprost at 10-4 M concentration (p=0,048). Although there was an uptrend in anti-angiogenic effect at increasing concentrations of each drug, no significant differences were found between the anti-angiogenic scores of iloprost and dipyridamole at each concentrations (p>0.05), except at the highest concentration of dipyridamole (10-4 M vs 10-6 M – p=0,041).
Discussion
Angiogenesis is a normal physiologic response that is triggered when tissues are exposed to stressful conditions such as injury or ischemia [13]. Current studies have focused on how endothelial progenitors contribute to vessel growth in adults as well as during the embryo stage of life. Also, the endothelial progenitors are similar in the fully developed heart [14]. When the major source of arterial blood becomes occluded, tissue ischemia ensues and vessel collateralization occurs during angiogenesis as a result of the initial hypoxic insult. Significant blood pressure differences develop between well-perfused and non-perfused tissues. In the setting of hypoxia, monocyte activity increases and vascular smooth muscle cells proliferate and migrate due to increased local growth factor and proteinase release [15,16]. Several factors increase in hypoxic states including hypoxia inducible factors (HIF), cytokines, placental growth factor (PIGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and VEGF, which all promote angiogenesis and vessel collateralization [17].
Recent advances revealed the importance of therapeutic angiogenesis for promoting the development of vessel collateralization in ischemic tissue via growth factor regulation. Several therapeutic angiogenesis trials have promising results in promoting angiogenesis in the setting of ischemic heart diseases, but definitive findings have yet to be revealed [18,19]. Iloprost has been prescribed to treat patients with symptomatic ischemic vascular diseases in which angiogenesis is important for improving clinical outcomes. It is understood that Iloprost facilitates long-term vasodilatation and inhibition of platelet aggregation [20]. Iloprost also has other effects such as inhibiting leukocyte activation and adhesion, reducing endothelial permeability, and inhibiting TNF-a as well as vasospasms induced by leukotrienes and “Endothelium-derived Constricting Factor” [21-24]. Another therapeutic effect of iloprost is its antagonism of cytokine-induced inflammation in certain disorders of inflammation [7]. TNF-a has pro-angiogenic effects at low doses, while higher doses and longer exposure periods to TNF-a actually inhibit factors that prevent angiogenesis [25]. Also, increased vascular permeability is essential for angiogenesis, and pro-angiogenic cytokines induce the expression of angiogenic factors under hypoxic conditions [2,26,27]. Vasodilatation facilitates angiogenesis as it enhances perfusion in ischemic tissues. Unfortunately, the complex and thus far occult interactions between iloprost and its effects on angiogenesis have yet to be fully understood.
Dipyridamole is an antithrombotic agent that is principally utilized for the secondary prevention of thrombotic vascular disease, and it also delays peripheral arterial disease progression [6]. Although dipyridamole is considered to be an anti-anginal agent, it has also been utilized in maintaining coronary artery graft patency and valve replacement surgery for blood clotting prevention; however, there is no current evidence that supports the routine use of dipyridamole for these indications [28]. Few reports assert that dipyridamole may improve coronary artery collateralization during ischemic insults, but another report suggested that there is an absence of vascular proliferation in the CAM animal model under the influence dipyridamole [29,30]. As such, the benefits of dipyridamole as a therapeutic agent in modulating angiogenesis remain largely unknown.
We studied the anti-angiogenic potential of iloprost and dipyridamole in the CAM animal model. Iloprost demonstrated no anti-angiogenic effect at 10-4 M, 10-5 M and 10-6 M concentrations. However, dipyridamole exhibited weak anti-angiogenic properties at the 10-4 M concentration, but no anti-angiogenic effects were observed at 10-5 M and 10-6 M concentrations.
Conclusion
The optimal therapeutic agent is one that provides adequate vascular vasodilatation without inhibiting angiogenesis in cardiovascular medicine. Our findings suggest that in the CAM chick model, both iloprost and dipyridamole demonstrated no significant anti-angiogenic properties, even though dipyridamole exhibited very weak anti-angiogenic effects. As such, these data support that these agents can be safely utilized to treat vascular diseases that cause tissue ischemia. We recommend that studies be performed in the future to further explore whether iloprost and dipyridamole have pro-angiogenic effects.
References
- Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 2002; 360: 427-435.
- Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation 2004; 109: 2487-2491.
- Chen YC, Chen CH, Ko WS, Cheng CY, Sue YM, Chen TH. Dipyridamole inhibits lipopolysaccharideinduced cyclooxygenase-2 and monocyte chemoattractant protein-1 via heme oxygenase-1-mediated reactive oxygen species reduction in rat mesangial cells. Eur J Pharmacol 2011; 650: 445-450.
- Grant SM, Goa KL. Iloprost. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peripheral vascular disease, myocardial ischaemia and extracorporeal circulation procedures. Drugs 1992; 43: 889-924.
- Wigley FM, Seibold JR, Wise RA, McCloskey DA, Dole WP. Intravenous iloprost treatment of Raynaud's phenomenon and ischemic ulcers secondary to systemic sclerosis. J Rheumatol 1992; 19: 1407-1414.
- Venkatesh PK, Pattillo CB, Branch B, et al. Dipyridamole enhances ischaemia-induced arteriogenesis through an endocrine nitrite/nitric oxide-dependent pathway. Cardiovasc Res 2010; 85: 661-670.
- Fiessinger JN, Schäfer M. Trial of iloprost versus aspirin treatment for critical limb ischaemia of thromboangiitis obliterans. The TAO Study. Lancet 1990; 335: 555-557.
- Norrby K. In-vivo models of angiogenesis. J Cell Mol Med 2006; 10: 588-612.
- Narayana A, Kelly P, Golfinos J, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg 2009; 110: 173-180.
- Dogan OT, Polat ZA, Karahan O, et al. Antiangiogenic activities of bemiparin sodium, enoxaparin sodium, nadroparin calcium and tinzaparin sodium. Thromb Res. 2011; 128: 29-32.
- Demirci B, Dadandi MY, Paper DH, Franz G, Baser KH. Chemical composition of the essential oil of Phlomis linearis Boiss. & Bal., and biological effects on the CAM assay: a safety evaluation. Z Naturforsch C 2003; 58: 826-829.
- Bobek V, Kovarik J. Antitumor and antimetastatic effect of warfarin and heparins. Biomed Pharmacother 2004; 58: 213-219.
- Carmeliet P. Angiogenesis in health and disease. Nat Med 2003; 9: 653-660.
- Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF). Proc Natl Acad Sci U S A 2002; 99: 8219-8224.
- Kamihata H, Matsubara H, Nishiue T, et al. Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscler Thromb Vasc Biol 2002; 22: 1804-1810.
- Heil M, Ziegelhoeffer T, Pipp F, et al. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol 2002; 283: 2411-2419.
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 2003; 9: 677-84.
- Henry TD. Therapeutic angiogenesis. BMJ 1999; 318: 1536-1539.
- Ng YS, D'Amore PA. Therapeutic angiogenesis for cardiovascular disease. Curr Control Trials Cardiovasc Med 2001; 2: 278-285.
- Baysal A, Bilsel S, Bulbul OG, Kayacioglu I, Idiz M, Yekeler I. Comparison of the usage of intravenous iloprost and nitroglycerin for pulmonary hypertensionduring valvular heart surgery. Heart Surg Forum 2006; 9: 536-542.
- Belch JJ, Saniabadi A, Dickson R, Sturrock RD, Forbes CD. Effect of iloprost (ZK 36374) on white cell behavior IN:Prostacyclin and its stable analogue iloprost, Gryglewski RJ and Stock G. Berlin- Heidelberg, Springer-Verlang 1987, 97-102.
- Lowe G. Critical Ischemia 1991; 1: 11-23.
- Scott JP, Higenbottam T, Wallwork J. The acute effect of synthetic prostacyclin analogue iloprost in primary pulmonary hypertension. Br J Clin Pract 1990; 44: 231-234.
- Stürzebecher CS. Effects of iloprost on platelet activation in vitro IN: Prostacyclin and its stable analogue iloprost, Gryglewski RJ and Stock G, Berlin- Heidelberg, Springer-Verlang 1987, 39-45.
- Ligresti G, Aplin AC, Zorzi P, Morishita A, Nicosia RF. Macrophage-derived tumor necrosis factor-alpha is an early component of the molecular cascade leading to angiogenesis in response to aortic injury. Arterioscler Thromb Vasc Biol 2011; 31: 1151-1159.
- Orlova VV, Economopoulou M, Lupu F, Santoso S, Chavakis T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VEcadherin-mediated cell-cell contacts. J Exp Med 2006; 203: 2703-2714.
- Dudek AZ, Mahaseth H.Circulating angiogenic cytokines in patients with advanced non-small cell lung cancer: correlation with treatment response and survival. Cancer Invest 2005; 23: 193-200.
- Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy-I. Prevention of death, myocardial infarction and stroke by prolonged antiplatelet therapy in various categories of patients. Br Med J 1994; 308: 81-106.
- Picano E, Michelassi C. Chronic oral dipyridamole as a ’novel’ antianginal drug: the collateral hypothesis. Cardiovasc Res 1997; 33: 666-670.
- Dusseau JW, Hutchins PM, Malbasa DS. Stimulation of angiogenesis by adenosine on the chick chorioallantoic membrane. Circ Res 1986; 59: 163-170.