Research Article - Biomedical Research (2016) Volume 27, Issue 4
Establishment of acute lung injury model in neonatal SD rats
Ying-ying Liu1,2#, Shao-bing Li1#*, Jin-hui Hu2, Rong Wu2#*1Basic Medical College, Anhui Medical University, Hefei 230032, PR China
2Neonatal Medical Center, Huaian Maternity and Child Healthcare Hospital, Yangzhou University Medical School, PR China
#Contribute equally to this work
- *Corresponding Authors:
- Rong Wu
Neonatal Medical Center
Huaian Maternity and Child Healthcare Hospital
Yangzhou University Medical School PR. China
Accepted on April 14, 2016
Abstract
Aim: The study is to understand histopathological abnormalities in neonatal Sprague-Dawley (SD) rats with acute lung injury (ALI) induced by different doses of lipopolysaccharide (LPS) and to explore suitable doses of LPS to develop ALI model of neonatal SD rats in 12-48 hours (h).
Methods: (1) 180 neonatal SD rats were randomly assigned into one of six groups: LPS 1mg group, LPS 1.5 mg group, LPS 2 mg group, LPS 2.5 mg group, LPS 3 mg group and control group (n=30 for each group). The rats were inoculated intraperitoneally with 1, 1.5, 2, 2.5, 3 mg/kg LPS and NS. We observed survival rate in each group within 48h. (2) 72 newly-born SD rats were randomly divided into LPS and control groups according to the above method (n=12 for each group). Each group rats were killed at 24, 36, and 48 h. Lungs were collected and used for histological analysis, histological score, determination of wet-dry ratio and radial alveolar cunt (RAC).
Results: (1) The rats in the control group alive all the time. However, the 24-36 h survival rate in the LPS groups were 67 %, 40 %, 23 %, 10 % and 0 respectively; The 48 h survival rate were 33.3%, 6.7%, 0, 0 and 0. (2) LPS aggravated degrees of ALI in neonatal SD rats in a dose-dependent manner. With increasing the dose of LPS and prolonged time, histological score and the ratio of W/D were increased, RAC count was decreased.
Conclusion: The 2, 2.5 mg/kg of LPS induced ALI model of neonatal SD rats might be used to study for the ALI within 24 h. The 1, 1.5 mg/kg of LPS induced ALI model of neonatal SD rats might be used to study for the ALI in 24-36 h. The 1 mg/kg of LPS induced ALI model of neonatal SD rats might be used to study for the ALI in 36-48 h.
Keywords
Acute lung injury, Animal model, Neonatal rat, Lipopolysaccharide
Introduction
Acute lung injury and acute respiratory distress syndrome (ALI/ARDS) are serious clinical disorders characterized by the disruption of the capillary-alveolar barrier, leading to the development of pulmonary edema and acute respiratory failure [1]. It often occurs in sepsis and in hemorrhagic shock [2]. It is one of the most common and fatal complication in neonates. ALI/ARDS is a severe inflammatory process of the lung. However, the molecular mechanisms responsible for the development of these conditions are poorly understood. Consensus criteria have been reached so that this clinical condition is now referred to as ARDS [1].
Despite multiple new treatment strategies have been introduced, the incidence and mortality of this disease remain unacceptably high [3]. The frequency of ALI in the United States alone is ~190,000 cases/year with a result of 74,500 deaths. ARDS mortality remains high at 36% to 44% [4,5].
It has been reported that sepsis, especially gram negative bacterial infection, is the most common cause of ALI/ARDS in neonates [6]. LPS, a major component of gram negative bacteria cell walls, was usually used to develop ALI in animals [7]. Hence, systemic administration of LPS has been widely used as a relevant model of ALI. Animal models have the potential to elucidate the mechanisms of disease and identify prognostic markers and therapeutic targets.
Recent studies found that the doses of 10 mg/kg and 5 mg/kg LPS administered respectively by intratracheal instillation could induce adult lung injury model. This results indicate that model of LPS-induced ALI is well established [8,9]. However, there hasn’t a complete and systematic report about developing ALI model of neonatal SD rats. Therefore, it will be meaningful for related research to establish the model of ALI with neonatal Sprague-Dawley (SD) rats.
The aim of this study was to understand histopathological abnormalities in neonatal SD with ALI induced by different doses of LPS and to explore suitable doses of LPS to develop ALI model of neonatal SD rats in 12-48 h.
Materials and Methods
Animals
Male and female SD rats (weighing 20 ± 2 g, age 10 days; from Anhui Medical University experimental animal center) were used. All experimental protocols were approved by the Institutional Animal Care and Use Committee at Anhui Medical University (permit number: 20150106).
Experimental design
(1) 150 newly-born SD rats were randomly assigned into one of five groups: LPS 1 mg group, LPS 1.5 mg group, LPS 2 mg group, LPS 2.5 mg group and LPS 3 mg group (n=30 for each group). Each group rats were inoculated intraperitoneally with 1, 1.5, 2, 2.5, 3 mg/kg of LPS was prepared from Escherichia coli O111:B6 (Sigma, St Louis, MO, USA), In addition to 30 newborn SD rats were inoculated intraperitoneally with normal saline (NS) as control group. We observed survival rate and general situation in each group within 48 h.
(2) 72 newly-born SD rats were randomly divided into LPS and control groups according to the above method (n=12 for each group). The LPS groups of animals were killed at 24, 36, and 48 h. Lungs were collected and used for histological analysis, histological score, determination of wet-dry ratio and radial alveolar cunt (RAC). Control group of animals were treated according to exactly the same protocol except that they received an intraperitoneal injection of NS instead of LPS.
Survival rate and general situation
The animals were fed milk and water. We monitored and recorded survival rate of each group in 48 h. At the same time, we also observed changes of respiratory ability and activity in new-born rats.
Histopathology analysis
The left lung tissues from twelve animals in each group were taken for histopathology. To harvest the lungs, we fixed the lungs with 4% paraformaldehyde. After overnight fixation, tissue was embedded in paraffin, sectioned, and stained with haematoxylin and eosin (HE). HE stains were made to determine morphology and inflammatory infiltrate. Paraffinembedded lungs were cut into 4 μm thick sections were visualized by light microscopy (400×) (Olympus, DP73) for histological analysis. In brief, ALI was classified into 4 categories based on the severity of alveolar congestion and hemorrhage, infiltration of neutrophils in the air spaces or vessel walls, and the thickness of alveolar wall/hyaline membrane formation. The severity of each category was graded from 0 (minimal) to 4 (maximal) and the total score was calculated by adding the scores in each of these categories. In each animal, 4 separate lung sections were graded to generate the mean score [10].
Wet-dry analysis
Wet-dry ratio (W/D) was measured as described previously [11]. The right upper lobes of lungs were removed immediately from newborn rats to prevent evaporative fluid loss from the tissues, then placed in a tarred microcentrifuge tube, and weighed. Lungs were then desiccated under a vacuum at 60°C for 72 h and weighed again. The wet lung mass was divided by the dry lung mass to give the wet-dry ratio.
Alveolar morphometry analysis
Alveolar development was evaluated by the RAC method as described previously with human lungs [12]. No counts were made if the respiratory bronchiole was nearer to the edge of the slide than to the nearest connective tissue septum. At least four different sections from each lung were used for this measurement. Photographs from six random 100× lung fields were taken from each section.
Statistical analysis
In the survival study, Survivals were compared by the Pearson Chi-square test. Continuous data were summarized as mean ± standard deviation (SD). Differences among all groups at each time point were analyzed by one-way analysis of variance (ANOVA) or Student’s t test. For comparisons between groups, LSD or Tamhane’s T2 with or without homegeneity of variances were used. P value less than 0.05 was considered statistically significant. Data were analysed using SPSS17.0 software (SPSS Inc, Chicago, IL, USA).
Results
Survival rate and general situation
As shown in Table 1, all rats in the control group alive no matter at 24, 36 or 48 h. However, the LPS groups displayed significantly shorter survival time. The 24h survival rate in the LPS groups were 100%, 100%, 100%, 100% and 65% respectively. The 36 h survival rate in the LPS groups were 67%, 40%, 23%, 10% and 0. At 48 h, rats in the LPS 2, 2.5, 3 mg groups all died, in addition to the LPS 1, 1.5 mg group, with 33.3%, 6.7% survival rate. Obviously, the 24, 36, 48 h survival rate were lower in the LPS groups than in the control group (all P<0.05). New-born rats in the LPS groups appeared activity decreased, unresponsive, breathing fast or difficulty, etc. With increased dose of LPS, the degree of difficulty in breathing was aggravated.
| Group | 24-36h | 36-48h | after 48h |
| Control group | 30 | 30 | 30 |
| 1mg group | 20a | 10a | 8a |
| 1.5mg group | 12a | 2a | 0a |
| 2mg group | 7ab | 0ab | 0a |
| 2.5mg group | 3abc | 0ab | 0a |
| 3mg group | 0abcd | 0ab | 0a |
| χ2 | 102.75 | 127.88 | 133.70 |
| P | 0.000▲ | 0.000▲ | 0.000▲ |
acompare with control group P<0.05; bcompare with 1 mg group P<0.05; ccompare with 1.5 mg group P<0.05; d compare with 2 mg group P<0.05. ▲: Fisher exact test
Table 1. The number of survivals in each group with neonatal SD rats in specific time (n=30).
Histological examination
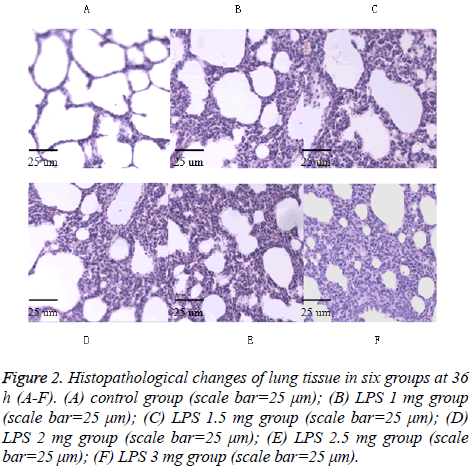
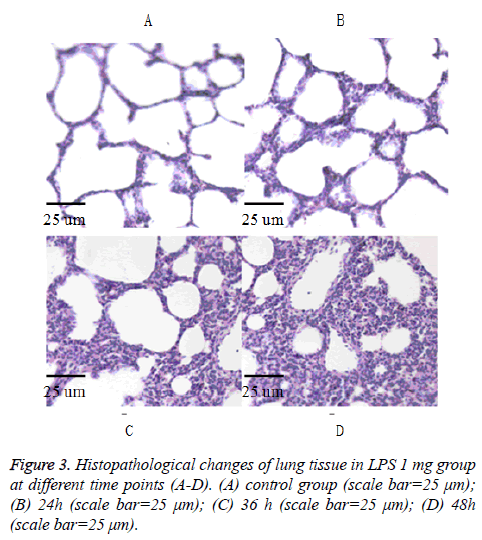
As shown in Figures 1-3 in the control group, observing the lung tissue slice of HE staining, secretion and inflammatory cell infiltration were not observed in the pulmonary alveolus cavity, and the structure of pulmonary alveolus wall is normal. In the LPS groups, acute lung damage with interstitial edema, hemorrhage, thickening of the alveolar wall, and infiltration of inflammatory cells into the interstitium and alveolar spaces were observed under the light microscope. In the LPS groups, the inflammatory cell infiltration and the degree of hemorrhage were increased and the alveolar septum edema was gradually thickened along with increased dose of LPS and prolonged time. The lung histological scores in all LPS groups were higher than in the control group at 24 h and 36 h (all p<0.05). The total histological scores in the LPS 1 mg group at each time point were higher than in the control group (all P<0.05) (Table 2).
Figure 1 : Histopathological changes of lung tissue in six groups at 24 h (A-F). (A) control group (scale bar=25 μm); (B) LPS 1 mg group (scale bar=25 μm); (C) LPS 1.5 mg group (scale bar=25 μm); (D) LPS 2 mg group (scale bar=25 μm); (E) LPS 2.5 mg group (scale bar=25 μm); (F) LPS 3 mg group (scale bar=25 μm).
Figure 2: Histopathological changes of lung tissue in six groups at 36 h (A-F). (A) control group (scale bar=25 μm); (B) LPS 1 mg group (scale bar=25 μm); (C) LPS 1.5 mg group (scale bar=25 μm); (D) LPS 2 mg group (scale bar=25 μm); (E) LPS 2.5 mg group (scale bar=25 μm); (F) LPS 3 mg group (scale bar=25 μm).
| Group | 24h after injection | 36h after injection | 48h after injection | F/t | P |
|---|---|---|---|---|---|
| Control group | 0.71±0.93 | 0.78±0.90 | 0.84±0.14 | 1.39 | 0.298 |
| 1mg group | 1.91±0.11a | 1.90±0.90a | 2.10±1.14a | 2.39 | 0.148 |
| 1.5mg group | 2.25±0.11a | 2.42±0.14ab | - | -1.90 | 0.107 |
| 2mg group | 2.78±0.16abc | 2.81±0.14abc | - | -0.26 | 0.801 |
| 2.5mg group | 3.50±0.27abc | 3.75±0.29abcd | - | -1.37 | 0.220 |
| 3mg group | 5.19±0.49abcde | 5.75±0.14abcde | - | -2.36 | 0.085 |
| F | 170.94 | 515.60 | -12.15 | ||
| P | 0.000 | 0.000 | 0.000 | ||
| -: Missing Values acompare with control group P<0.05; bcompare with1mg group P<0.05; ccompare with1.5 mg group P<0.05; dcompare with 2 mg group P<0.05;ecompare with 2.5 mg group P<0.05 |
|||||
Table 2. The changes of lung histological score in each group with neonatal SD rats at different time points ( , n=4).
, n=4).
Determination of lung water
The lung water content was higher in the LPS groups than in the control group, and the higher dose of LPS, the higher lung water content. There was statistically difference between the LPS groups and the control group (all P<0.05) (Table 3).
| Group | 24h after injection | 36h after injection | 48h after injection | F/t | P |
|---|---|---|---|---|---|
| Control group | 1.49±0.19 | 1.59±0.43 | 1.70±0.02 | 3.31 | 0.084 |
| 1mg group | 1.76±0.07a | 1.82±0.06a | 1.91±0.05 | 5.90 | 0.023 |
| 1.5mg group | 2.03±0.11ab | 2.27±0.11ab | - | -3.06 | 0.022 |
| 2mg group | 2.69±0.11abc | 3.11±0.12abc | - | -5.26 | 0.002 |
| 2.5mg group | 3.96±0.20abcd | 4.15±0.18abcd | - | -1.38 | 0.217 |
| 3mg group | 4.41±0.13abcde | 4.74±0.17abcde | - | -3.13 | 0.022 |
| F | 283.63 | 426.70 | -8.09 | ||
| P | 0.000 | 0.000 | 0.002 | ||
| -: Missing Values acompare with control group P<0.05; bcompare with1mg group P<0.05; ccompare with1.5 mg group P<0.05; dcompare with 2 mg group P<0.05;ecompare with 2.5 mg group P<0.05 |
|||||
Table 3. Lung tissue W/D value in each group with neonatal SD rats at different time points ( ± s, n=4 ).
± s, n=4 ).
Alveolar morphometry
The RAC of lung sections in the LPS groups were lower than in the control group. The RAC were decreased with increased dose of LPS and prolonged time. There was statistically difference between the LPS groups and the control group (all P<0.05) (Table 4).
| Group | 24 h after injection | 36 h after injection | 48h after injection | F/t | P |
|---|---|---|---|---|---|
| Control group | 12.75±1.14 | 12.94±1.07 | 12.38±0.48 | 0.37 | 0.701 |
| 1mg group | 10.88±0.32 | 10.44±1.00 | 9.62±0.72a | 2.94 | 0.104 |
| 1.5mg group | 8.50±0.35ab | 7.81±0.69a | - | 1.78 | 0.126 |
| 2mg group | 6.00±0.46abc | 5.56±0.43abc | - | 1.40 | 0.211 |
| 2.5mg group | 4.19±0.24abcd | 3.81±0.31abcd | - | 1.90 | 0.107 |
| 3mg group | 2.81±0.24abcde | 2.31±0.24abcde | - | 2.95 | 0.025 |
| F | 195.63 | 132.26 | 6.35 | ||
| P | 0.000 | 0.000 | 0.001 | ||
| -: Missing Values acompare with control group P<0.05; bcompare with1mg group P<0.05; ccompare with1.5 mg group P<0.05; dcompare with 2 mg group P<0.05;ecompare with 2.5 mg group P<0.05 |
|||||
Table 4. The dynamic changes of RAC in each group with neonatal SD rats at different time points ( , n=4).
, n=4).
Discussion
The choice of ALI animal model for evaluation of innovative therapeutic interventions and mechanism research is critical due to the complexity of ALI, and the apparent disconnect between animal and clinical studies [13]. Currently, the animal models of ALI can be induced by hydrochloric acid, repeated saline lavage, oleic acid and LPS, etc. They have strengths and weaknesses of different types of animal models that have been used to study the mechanisms and treatment of ALI [14]. Owning to gram-negative sepsis is one of the most common causes of ALI/ARDS in humans; the model of LPS-induced ALI has clinical relevance [7]. The pathophysiology in the LPS animal model is consistent with an elevated inflammatory response. It is also very reproducible and provides important information about host inflammatory responses [15] and it is not only appropriate for studying the pathogenesis of ALI/ ARDS but also for studying ALI/ARDS complicated with heart failure or pulmonary hypertension [14].
The pathophysiology of ALI/ARDS progresses through three overlapping stages consisting of an early exudative stage, amid proliferative stage, and a late fibrotic stage [16]. The exudative stage begins at 12 to 24 hours after the initiating insult and may continue through 7 days; The next pathophysiologic stage is the proliferative, which may begin 3 days after the original insult and can last as long as 2 week; The last stage of ALI/ ARDS injury is the fibrotic, which lasts 5 to 28 days after injury. It is reported that the clinical manifestations of ALI develop acutely, usually within 24 to 48 h after the initiating insult [17-19]. Therefore, survival rate combined with lung injury’s degree are essential for the study of ALI model within 48 h underlying the development of this severe disease.
Our results showed the pathology of the lung is consistent with an elevated inflammatory response of patients with ALI. In the LPS groups, interstitial edema, haemorrhage, thickening of the alveolar wall, and infiltration of inflammatory cells into the interstitium and alveolar spaces were observed under the light microscope. These results demonstrate that LPS could induce injury and inflammatory response in the lung, suggesting that LPS-induced ALI was successfully established in neonatal rats; it is consistent with findings of An JF et al. [20].
LPS play a key role in inducing ALI, and the dose of LPS is an important factor in the degree of ALI. In adult rats, the researchers reported that mild ALI model was induced in Wistar rats by intraperitoneal LPS (5 mg/kg) and arterial blood gas were recorded between 24 h and 25 h after the injection of LPS [21]. In neonatal rats, the investigators reported that ALI model were established by intraperitoneal LPS (3 mg/kg), the general conditions of the rats were observed and samples were collected at 24 h after the injection of LPS, just before their sacrifice [15]. This study showed that in the 24 h after the injection of LPS, all newborn rats alive in LPS groups except for 3 mg group, the survival rate is only 65%. This result showed that intraperitoneal injection of 3 mg/kg LPS causes severe lung injury and survival rate is low at 24 h, suggesting 3 mg/kg is not a suitable dose to develop model. Therefore, the doses of 2, 2.5 mg/kg are undoubtedly the best choice according to survival rate and the degree of lung injury; In the 36 h, the survival rate in the LPS groups were 67%, 40%, 23%, 10% and 0 respectively. Due to lower survival rate and severe degree of lung injury in the 2, 2.5, 3 mg groups, the doses of 2, 2.5, 3 mg/kg are not suitable for study. Therefore, the dose of 1, 1.5 mg/kg is undoubtedly the best choice according to survival rate and the degree of lung injury; in the 48 h, newborn rats all died in LPS groups except for 1, 1.5 mg group, the survival rate are 33.3% and 6.7%. Survival rate of 1.5 mg group is too low; suggesting the dose of 1 mg/kg is the best choice if you want to observe ALI longer time. However, the study reported that the survival rate of the Wistar Rats is 50% (8/16) at 16 h and 29.2% (7/24) at 24 h after intraperitoneal LPS (5 mg/kg) [22]. This is not consistent with our results. The possible reason may due to the species of rat.
Conclusions
The different doses (1, 1.5, 2, 2.5, 3 mg/kg) of LPS can prepare ALI model in neonatal rats, and the degree of ALI model was aggravated with increasing dose of LPS. The 2, 2.5 mg/kg of LPS induced ALI model of neonatal SD rats might be used to study for the ALI within 24 h. The 1, 1.5 mg/kg of LPS induced ALI model of neonatal SD rats might be used to study for the ALI in 24-36 h. The 1 mg/kg of LPS induced ALI model of neonatal SD rats might be used to study for the ALI in 36-48 h.
Acknowledgements
This research was funded by grants of Key Child Health Personnel in Jiangsu Province (Project no: FRC201211).
References
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307: 2526-2533.
- Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J RespirCrit Care Med 1995; 151: 293-301.
- Cheifetz IM. Pediatric acute respiratory distress syndrome. Respir Care 2011; 56: 1589-1599.
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353: 1685-1693.
- Tsushima K, King LS, Aggarwal NR, De Gorordo A, D'Alessio FR, Kubo K. Acute lung injury review. Intern Med 2009; 48: 621-630.
- Fein AM, Calalang-Colucci MG. Acute lung injury and acute respiratory distress syndrome in sepsis and septic shock. Crit Care Clin 2000; 16: 289-317.
- Krupa A, Fol M, Rahman M, Stokes KY, Florence JM, Leskov IL, Khoretonenko MV, Matthay MA, Liu KD, Calfee CS, Tvinnereim A, Rosenfield GR, Kurdowska AK. Silencing Bruton's tyrosine kinase in alveolar neutrophils protects mice from LPS/immune complex-induced acute lung injury. Am J Physiol Lung Cell MolPhysiol 2014; 307: L435- L448.
- Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J RespirCrit Care Med 2006; 173: 1008-1015.
- Izushi Y, eshigawara K, Liu K, Wang D, Wake H, Takata K, Yoshino T, Takahashi HK, Mori S, Nishibori M. Soluble form of the receptor for advanced glycation end-products attenuates inflammatory pathogenesis in a ratmodel of lipopolysaccharide-induced lung injury. J PharmacolSci 2016.
- Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymalstemcells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 2007; 179: 1855-1863.
- Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. Journal of Immunology 2007; 178: 8127-8137.
- Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1-postnatal lung growth. Thorax 1982; 37: 572-579.
- Bastarache JA, Blackwell TS. Development of animal models for the acute respiratory distress syndrome. Dis Model Mech 2009; 2: 218-223.
- Rosenthal C, Caronia C, Quinn C, Lugo N, Sagy M. A comparison among animal models of acute lung injury. Crit Care Med 1998; 26: 912-916.
- Li Y, Wu R, Tian Y. Yu M, Tang Y, Cheng H, Tian Z. RAGE/NF-κBsignaling mediates lipopolysaccharide induced acute lung injury in neonate rat model. Int J ClinExp Med 2015; 8: 13371-13376.
- Mendex JL, Hubmayr RD. New insights into the pathology of acute respiratory failure. CurrOpinCrit Care 2005; 11: 29-36.
- Walkey AJ, Summer R, Ho V, Alkana P. Acute respiratory distress syndrome: epidemiology and management approaches. ClinEpidemiol 2012; 4: 159-169.
- Bream-Rouwenhorst HR, Beltz EA, Ross MB, Moores KG. Recent developments in the management of acute respiratory distress syndrome in adults. Am J Health Syst Pharm 2008; 65: 29-36.
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342: 1334-1349.
- An JF, Sun Y, Zhang QL, Zhang FL, Zhang JL. The effects of germacrone on lipopolysaccharide-induced acute lung injury in neonatal rats. Cell MolBiol (Noisy-le-grand) 2014; 60: 8-12.
- Bianchi AM, Reboredo MM, Lucinda LM, Reis FF, Silva MV, Rabelo MA, Holanda MA, Oliveira JC, Lorente JÁ, PinheiroBdo V. The Effects of Prone Position Ventilation on Experimental Mild Acute Lung Injury Induced by Intraperitoneal Lipopolysaccharide Injection in Rats. Lung 2016; 194: 193-199.
- Du Y, Wu YB, Cai XX, Han YK. Changes of platelet endothelial cell adhesion molecule-1, tissue type plasminogen activator and plasminogen activator inhibitor-1 expression in the lung tissue of neonatal rats after intraperitoneal injection with lipopolysaccharide. ZhonghuaErKeZaZhi 2004; 42:649-653.