Research Article - Biomedical Research (2017) Volume 28, Issue 14
Establishment and evaluation of rat epilepsy model by basolateral amygdala kindling
Guanshui Bao1*#, Xuqin Chen2#, Ying Hua3, Zhedong Wang2 and Fei Ye41Department of Neurology, Shanghai 9th People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, PR China
2Department of Neurology, Affiliated Children's Hospital of Soochow University, Suzhou, Jiangsu, PR China
3Department of Child Neurology, Wuxi People's Hospital, Wuxi, Jiangsu, PR China
4Department of Neurology, Jinhua Municipal Central Hospital, Jinhua, Zhejiang, PR China
#These authors contributed equally
- *Corresponding Author:
- Guanshui Bao
Department of Neurology
Shanghai 9th People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, PR China
Accepted date: June 15, 2017
Abstract
This study aims to establish a rat epileptic model by basolateral amygdala (BLA) kindling and evaluate its feasibility. Forty male rats were randomly divided into blank group (5 rats; no electrode insertion), sham-operated group (5 rats; the electrodes were inserted in BLA, without electrical stimulation) and kindling group (30 rats; the electrodes were inserted in BLA; the electrical stimulation was performed every day, until reaching the fully kindled state). During the experiment, the video electroencephalograph was synchronously recorded, and the behavior changes were observed. The pathological changes of hippocampus and the expressions of phosphorylated c-Jun N-terminal kinase (P-JNK) in hippocampus were determined. Results showed that, 24 of 29 stimulated rats were successfully kindled, with success rate of 82.8% (24/29). In 24 kindled rats, the grade-5 epilepsy attack occurred on the 8.29 ± 5.31 day, and the kindled state was achieved on the 10.87 ± 3.44 day. No rat died due to epilepsy attack. The after-discharge duration was 32.05-51.65 s. The kindled rats were more irritable than those in the sham-operated group. There was obvious neuronal loss and glial proliferation in hippocampus in kindling group. The expression of P-JNK in hippocampus in kindling group was significantly higher than blank and sham-operated group, respectively (P<0.05). The rat epileptic model by BLA kindling is successfully established. It may be a good tool for investigating the epilepsy mechanism, prevention and treatment.
Keywords
Epilepsy, Rat, Kindling, Model, Basolateral amygdala.
Introduction
Epilepsy model by amygdala kindling is one of the chronic epilepsy models which have high-degree similarity with the occurrence of human epilepsy. In 1969, Goddard et al. [1] used 60 Hz electric pulse to stimulate the amygdala of rat once a day. After two weeks, the epilepsy was successfully induced, and this phenomenon was called as kindling effect. After decades of research, it is found that the epilepsy model by amygdala kindling can sustain for several months to several years once it is established, and its epileptic behaviors are normative. The electrode position can be set by the experimenter, thus the epilepsy focus position and attack time can be controlled randomly, and the repeatability of model is good. In addition, the animals have a good response to stimulation, and the mortality rate is low. This model accords with the most conditions of the ideal animal epilepsy models, and has much more advantages than the epilepsy model which is induced by drugs [2]. With the development of video electroencephalograph (EEG) and long-term dynamic EEG, it is easy to better observe the spontaneous recurrent seizure [3]. Therefore, the epilepsy model by amygdala kindling is widely used in studying the epilepsy behaviors [4,5].
Amygdala is a complex tissue. In most epilepsy models by amygdala kindling, the electrical stimulation is only located at amygdala. It is relatively rough, and the experimental errors are large. Basolateral amygdala (BLA) has the most extensive conduction bundles which are related to the emotion of fear and anxiety [6]. In this study, the rat epileptic model by BLA kindling was established. The electroencephalograph (EEG) of BLA was recorded; the rat behavior changes and hippocampal pathological changes were observed; the expression of phosphorylated c-Jun N-terminal kinase (P-JNK) in hippocampal tissue was determined. The objective was to evaluate the feasibility of rat epileptic model by BLA kindling, and provide a basis for its further application.
Materials and Methods
Animals and grouping
Forty male Sprague-Dawley rats (provided by the Center for Experimental Animals of Suzhou University; 6 weeks age; 180-280 g; single-cage raised in the condition avoiding strong light and noise; 12/12 h day-night cycle; with free access to food and water) were randomly divided into blank group (5 rats; no electrode insertion; caught once a day), sham-operated group (5 rats; the electrodes were inserted in BLA, without electrical stimulation) and kindling group (30 rats; the electrodes were inserted in BLA; the electrical stimulation was performed every day, until reaching the fully kindled state). The SEY-3201 electronic stimulator was provided by Shanghai Qichi Instrument Co., Ltd. (Shanghai, China).
Electrode insertion method
Rats were anaesthetize by intraperitoneal injection with 4% chloral hydrate (10 ml·kg-1; Sigma-Aldrich Corp., MO, USA). Then the rats were fixed on the stereotaxic instrument (Shaanxi Wandong Medical Equipment Co., Ltd., Xi’an, China). After determining the position of left BLA, the bipolar electrodes with 0.20 mm diameter were inserted (the electrode position could be confirmed by tissue sections at the end of the experiment). The stainless steel screws were fixed the right forehead, right head and occiput, respectively, which were also used as the recording electrodes. Above electrodes were fixed on the surface of skull using denture powder (Guangzhou Kesun Medical Equipment Co., Ltd., Guangzhou, China), and were connected to stimulator and EEG amplifier. After surgery, the penicillin sodium was intraperitoneally injected for 3 consecutive days for preventing the infection. Rats were recovered for 1 week after surgery.
Kindling process
BLA of rats was stimulated once a day according to the following parameters as follows: 400 μA single-phase square wave; wave width 1 ms; frequency 50 Hz; duration 2 s. The intensity of attack was observed. If it did not reach grade 5 according to grading in Racine's scale [7] after stimulating for more than 30 times, or it reached the grade 5, followed by gradual regression (could no longer reach grade 5), the stimulation was given up. The grading of attack intensity in Racine's scale was as follows: grade 0: EEG showed epilepsy attack waveform, without twitching action; grade 1: static, eyes closed, beard agitating, facial twitched; grade 2: noding, chewing, facial twitching; grade 3: unilateral forelimb lifting, clonus; grade 4: standing with bilateral forelimb clonus; grade 5: standing, falling, systemic clonic. When the seizure intensity reached grade 5 for consecutive 5 times, the kindling was considered successful, and the stimulation was stopped.
Video EEG synchronous recording and behavior observation
Cadwell video EEG analysis system (version 1.70; Cadwell Therapeutics Inc., WA, USA) was used to synchronously record the background EEG of rats in peacetime and the afterdischarge duration (ADD) by electrical stimulation. During the experiment, the emotional response of rats at peacetime and the defence response when catching were observed.
Pathological examination
After the epilepsy model by BLA kindling was established, the rats in kindling group and sham-operated group were perfused with 4% paraformaldehyde solution (Sigma-Aldrich Corp., MO, USA). The brain was taken, and was immersed in 4% paraformaldehyde solution for 24 h, followed by soaking and dehydrating with 30% sucrose solution (Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, China) till the sample sank to the bottom. The specimen was wrapped with aluminum foil, and was placed in the refrigerator at -80°C for use. The specimen was embedded with paraffin, followed by hematoxylin and eosin (HE) staining and observation. The related reagents were provided by Sigma-Aldrich Corp. (MO, USA).
Determination of P-JNK expression in hippocampal tissue
Rats were sacrificed, and the hippocampal tissue was extracted. The 10% SDS-PAGE was performed for 3 h to separate the protein. The expressions of total c-Jun N-terminal kinase (t- JNK) and P-JNK were determined by Western blot analysis according to the reported methods [8].
Statistical analysis
All statistical analysis was carried out using SPSS17.0 software (SPSS Inc., Chicago, IL, USA). The data were presented as mean ± SD, and were compared using t test. P<0.05 was considered as statistically significant.
Results
Outcome of BLA kindling
All 5 rats survived in blank group. In sham-operated group, 1 rat died during the electrode insertion operation. The remaining 29 rats were given with the electrical stimulation. There were 2 rats with no finding of after discharge or no twitch response. In 2 rats the after discharge and twitch response were observed, but the kindling was not successfully performed due to electrode shedding in the process of stimulation. In 1 rat the after discharge with grade 5 was recorded for 2 times, but the onset level gradually decreased in the later stimulation, and ultimately the kindling process failed. The kindling was successfully conducted in 24 rats, and the success rate was 82.8% (24/29).
Epilepsy attack in kindled rats
In 24 kindled rats, the grade-5 epilepsy attack occurred on the 8.29 ± 5.31 day, and kindled state was achieved on the 10.87 ± 3.44 day. Most of the rats had a typical twitch action. After the stage of wet-dog shaking, transient stillness or sniffing, the actions returned to normal. There was no rat which died due to epilepsy attack. After stimulation, partial rats appeared action of dramatic running, jumping, and then falling to the ground, with limbs clonus or paralysis, but these symptoms disappeared soon.
Synchronous recording result of video EEG
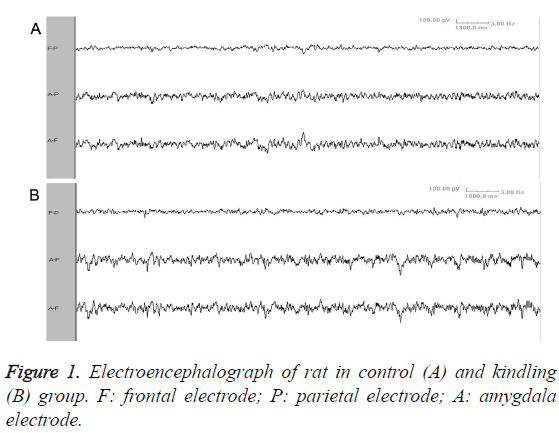
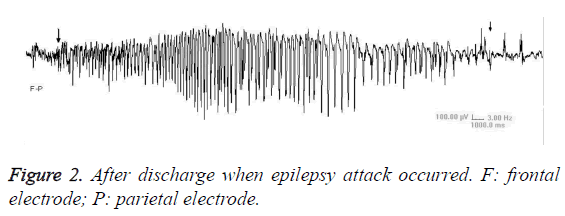
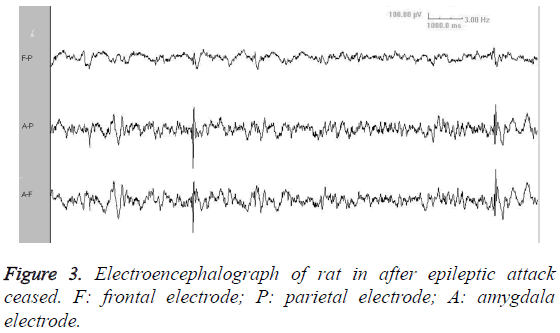
Video EEG showed that, there was no significant difference of background EEG between kindling group and sham-operated group. Compared with sham-operated group, the kindling group presented increased slow-wave rhythm and decreased wave amplitude (Figure 1). The ADD was 32.05-51.65 s (Figure 2). After a long period from epileptic attack ceasing, the slow-wave rhythm increased, with appearance of intermittent spike wave or shark spike wave (Figure 3), which synchronized with the wet-dog shaking action.
Behaviour changes
When partial rats in kindling group were placed in one cage, they immediately fought and bit each other. Compared with sham-operated group, the rats in kindling group were hard to catch, and most rats presented defensive posture by lifting and opening the bilateral forelimbs. There was no significant difference of behavior change between the blank group and sham-operated group.
Pathological findings in hippocampus
HE staining results showed that, in kindling group, the neurons in CA1, CA3 and amygdala region of hippocampus were sparse, with obvious neuronal loss and glial proliferation around electrode insertion site. The blank group and shamoperated group had no obvious pathological change in the electrode insertion area in amygdala.
Expressions of t-JNK and P-JNK in hippocampus
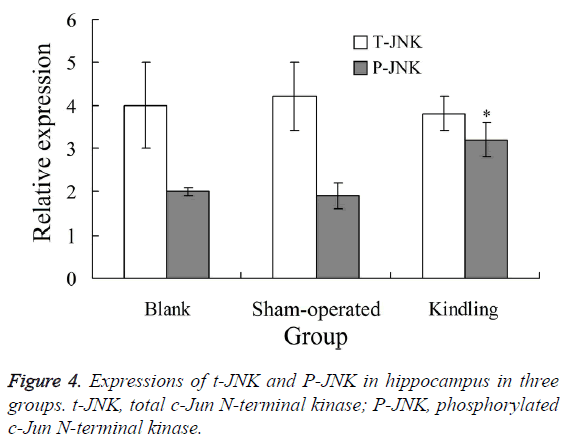
Western blot analysis showed that, there was no significant difference of t-JNK expression in hippocampus among blank, control and kindling group (P>0.05). The expression of P-JNK in hippocampus in kindling group was significantly higher than blank and sham-operated group, respectively (P<0.05). There was no significant difference of P-JNK expression between blank and sham-operated group (P>0.05) (Figure 4).
Discussion
Epilepsy is a disease which seriously threatens the human health. However, due to the particularity of brain, there is great difficulty in studying the epilepsy of human. Therefore, the research on epilepsy cannot be performed without using animal epilepsy models. There are more than 100 kinds of animal models of epilepsy [9]. The spontaneous limbic-system epilepsy induced by electric kindling is very similar with the human temporal lobe epilepsy in aspects such as semeiology, neuropathology and behavioral defects [10]. The amygdala kindling model is the only animal model which can be used to study drug-resistant epilepsy [9]. This model can also be used to determine whether epilepsy is a progressive disease or not [11]. The amygdala is a complex tissue. Although there is a small amount of electric kindling model, the electrical stimulation is only located at the amygdale. So the amygdala kindling model is relatively rough, and the experimental errors are large. The BLA has the most extensive conduction bundles, and the single-synaptic fibers are the most sensitive area in BLA, and radiate to all levels of the hippocampus [12]. The kindling of BLA can induce the increase of excitatory neurotransmitter and the decrease of γ-aminobutyric acid, which increases the excitability of neurons, and gradually forms the epileptogenic focus. The after discharge arisen by the electrical stimulation causes the synchronous discharge and spreading of brain neurons, which transfer to the contralateral amygdala [13].
In this study, the epilepsy in rats was kindled by chronic electrical stimulation of BLA, and the kindled sate was similar with that in the human temporal lobe epilepsy. The epilepsy rats presented impatient and aggressive action, and were easy to be caught. This is in line with the result of previous studies [14,15]. The kindled animals often have emotional and behavioral abnormalities, and they are often easy to be provoked, which is very meaningful for studying the mental behavior change with human epilepsy [16]. The video EEG showed that, the kindling group presented increased slow-wave rhythm and decreased wave amplitude, which was consistent to the EEG performance of human epilepsy. After a long period from induced epileptic attack, the slow-wave rhythm increased, with appearance of intermittent spike wave or shark spike wave. Some scholars [17] believe that, these waveform changes are related to the corresponding behavior changes. This is worth further investigation.
JNK is one member of MAPK super-family, which belongs to the evolutionarily conserved serine/threonine protein kinase. It can regulate the functional activity of various substrates by phosphorylation, and alter the gene expression [18]. JNK signaling pathway plays a crucial role in the cell differentiation, apoptosis, stress responses and occurrence and development of various human diseases [19]. It is considered as an important regulator target of cells in normal and disease states. Results of this study showed that, the expression of PJNK in hippocampus in kindling group was significantly higher than blank and sham-operated group, respectively (P<0.05). This indicates the abnormal increase of P-JNK level after kindling. In addition, the P-JNK expression level in hippocampal area increased significantly after the attack of epilepsy. The reason is that, the model established in this study is a chronic kindling model, in which the P-JNK level presents a repeated and continuous rise, but not just a transient change in the chemical kindling model.
In this study, there was obvious neuronal loss and glial proliferation in hippocampus in kindling group, which was accordant to the abnormal phosphorylation of JNK. This suggests that, after the repeated epilepsy attack, the complex stress response of nervous tissue is initiated; the JNK signaling pathway is activated, leading to the corresponding downstream target effect, e.g., promotion of reactive hyperplasia of glial cells. The reactive proliferation of glial cells can change the extracellular microenvironment of cells, so the normal contact among nerve cells is broken, resulting in abnormal discharge and epilepsy attack. This can form a vicious spiral, which eventually causes the occurrence of hippocampal sclerosis [20,21].
Establishment of kindling model will take more time and energy, which limits its application to a certain extent. Previous study [22] has developed some relatively energy-saving electric stimulation models such as corneal kindling model and acute focal seizure model, but these models still have some problems when applied to the study of anti-epileptic drugs. In conclusion, the rat epilepsy model by BLA kindling is very similar to the human epilepsy. It is convenient to study the epilepsy from aspect of behavior science, EEG, histology and biochemistry, and can avoid the direct toxic effects of chemical drugs on neurons. It may be a good tool for investigating the epilepsy mechanism, prevention and treatment.
Acknowledgement
This work was supported by the Scientific Research Project of Shanghai Science and Technology Committee (No. 14411972200), Scientific Research Project of Shanghai Health Bureau (No. 20134067) and Scientific and Technology Project of Jinhua Municipal Science and Technology Bureau (No. 2013-3-037).
References
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol 1969; 25: 295-330.
- Sarkisian MR. Overview of the Current Animal Models for Human Seizure and Epileptic Disorders. Epilepsy Behav 2001; 2: 201-216.
- Bertram EH, Williamson JM, Cornett JF, Spradlin S, Chen ZF. Design and construction of a long-term continuous video-EEG monitoring unit for simultaneous recording of multiple small animals. Brain Res Brain Res Protoc 1997; 2: 85-97.
- Arida RM, Scorza FA, dos Santos NF, Peres CA, Cavalheiro EA. Effect of physical exercise on seizure occurrence in a model of temporal lobe epilepsy in rats. Epilepsy Res 1999; 37: 45-52.
- Kalynchuk LE. Long-term amygdala kindling in rats as a model for the study of interictal emotionality in temporal lobe epilepsy. Neurosci Biobehav Rev 2000; 24: 691-704.
- Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, Carroll FI, Nestler EJ, Konradi C, Carlezon WA. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry 2011; 70: 425-433.
- Bolkvadze T, Pitkänen A. Development of post-traumatic epilepsy after controlled cortical impact and lateral fluid-percussion-induced brain injury in the mouse. J Neurotrauma 2012; 29: 789-812.
- Kizilay G, Cakmak H, Yen CF, Atabekoglu C, Arici A, Kayisli UA. Expression and regulation of c-Jun N-terminal kinase (JNK) in endometrial cells in vivo and in vitro. Histochem Cell Biol 2008; 130: 761-771.
- Löscher W. Animal models of intractable epilepsy. Prog Neurobiol 1997; 53: 239-258.
- Bertram EH. Functional anatomy of spontaneous seizures in a rat model of limbic epilepsy. Epilepsia 1997; 38: 95-105.
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 1997; 20: 517-523.
- Ebert U, Koch M. Acoustic startle-evoked potentials in the rat amygdala: effect of kindling. Physiol Behav 1997; 62: 557-562.
- Adamec R, Young B. Neuroplasticity in specific limbic system circuits may mediate specific kindling induced changes in animal affect-implications for understanding anxiety associated with epilepsy. Neurosci Biobehav Rev 2000; 24: 705-723.
- Velísek L, Velísková J, Stanton PK. Low-frequency stimulation of the kindling focus delays basolateral amygdala kindling in immature rats. Neurosci Lett 2002; 326: 61-63.
- Aker RG, Yananli HR, Gurbanova AA, Ozkaynakçi AE, Ateş N, van Luijtelaar G, Onat FY. Amygdala kindling in the WAG/Rij rat model of absence epilepsy. Epilepsia 2006; 47: 33-40.
- van Lier H, Drinkenburg WH, Coenen AM. Strain differences in hippocampal EEG are related to strain differences in behaviour in rats. Physiol Behav 2003; 78: 91-97.
- Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res 2001; 47: 217-227.
- Barr RK, Bogoyevitch MA. The c-Jun N-terminal protein kinase family of mitogen-activated protein kinases (JNK MAPKs). Int J Biochem Cell Biol 2001; 33: 1047-1063.
- Leppä S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene 1999; 18: 6158-6162.
- Richardson RM, Barbaro NM, Alvarez-Buylla A, Baraban SC. Developing cell transplantation for temporal lobe epilepsy. Neurosurg Focus 2008; 24: E17.
- Bordey A, Sontheimer H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res 1998; 32: 286-303.
- Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res 2001; 47: 217-227.