Research Article - Biomedical Research (2017) Volume 28, Issue 11
Epidural fibrosis post laminectomy is the unappreciated cause of spinal cord compression post chronic spinal cord injury
Xiuxin Han1#, Xiao Tian2#, Baklaushev VP3, Abakumov MA4, Patricia Douglas5, Haixiao Wu4, Ratyev AP6,7, Egiazaryan KA6,7, Lili Li1, Derun Tian5, Guowen Wang1, Xiubao Ren2*, Chekhonin VP4,8 and Chao Zhang1,4,8*
1Department of Bone and Soft Tissue Tumors, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin Medical University Cancer Institute and Hospital, Tianjin, PR China
2Department of Immunology, Key Laboratory of Cancer Immunology and Biotherapy, National Clinical Research Center of Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin, PR China
3Federal Research and Clinical Center, FMBA of Russia
4Department of Medicinal Nanobiotechnology, Pirogov Russian National Research Medical University, Moscow, Russian Federation
5Department of Anatomy, Tianjin Medical University Tianjin, PR China
6Department of Traumatology, Orthopaedics and Military Field Surgery, Pirogov Russian National Research Medical University, Moscow, Russian Federation
7Department of Traumatology Orthopaedics, 1 Clinical City Hospital, Moscow, Russian Federation
8Department of Basic and Applied Neurobiology, Federal Medical Research Center for Psychiatry and Narcology, Moscow, Russian Federation
#These authors contribute equally to this work
- *Corresponding Authors:
- Chao Zhang
Department of Bone and Soft Tissue Tumors
National Clinical Research Center for Cancer
Key Laboratory of Cancer Prevention and Therapy
Tianjin Medical University Cancer Institute and Hospital, PR China
- Xiubao Ren
Department of Immunology, Key Laboratory of Cancer Immunology and Biotherapy
National Clinical Research Center of Cancer
Tianjin Medical University Cancer Institute and Hospital, PR China
Accepted date: March 15, 2017
Abstract
Spinal cord injury is the result of extreme trauma to the spinal cord. The trauma often results in a series of lasting and significant symptoms which reduce patients’ quality of life. In the present research, thirty healthy adult Sprague-Dawley rats were randomly divided into three groups: (1) mild injury group (laminectomy and spinal cord impacting, force value=100 kdyn), (2) severe injury group (laminectomy and spinal cord impacting, force value=200 kdyn), (3) control group (laminectomy only). In order to prove the creation of spinal cord injury and epidural fibrosis within the animal model, BBB score tests were performed weekly and MRI scans were done at 4 weeks post-operation. Macroscopic evaluation and histology were used to evaluate the correlation between spinal cord injury and epidural fibrosis formation. Spinal cord injury can instigate epidural fibrosis. The higher grades of spinal cord injury were associated with more widespread epidural adhesion and greater spinal cord compression. Epidural fibrosis is an unappreciated cause of spinal cord compression post laminectomy in spinal cord injury rats. Epidural scarring has been noted to easily form in spinal cord injury model rats. The present study suggests the necessity of preventing epidural fibrosis post-surgery in patients with spinal cord injury.
Keywords
Spinal cord injury, Epidural fibrosis, Neuropathic pain.
Abbreviations
SCI: Spinal Cord Injury; EF: Epidural Fibrosis; FBSS: Failed Back Surgery Syndrome; BBB score: Basso, Beattie, and Bresnahan score; MRI: Magnetic Resonance Imaging.
Introduction
Spinal Cord Injury (SCI) is the result of an extremely serious physical trauma to the spinal cord. The lasting and significant impact including paralysis, gatism, hyperalgesia, hyperthermoesthesia and chronic pain, severely reduce the patients’ quality of life. The epidemiological study suggested the annual incidence of SCI reached 23.7 cases per million people in China [1].
Spinal cord swelling was reported to be correlated with worst outcome [2]. Spinal cord swelling develops during the first 72 h post SCI, with the combination of spinal cord haemorrhage and oedema [3,4]. Decompression by laminectomy, which creates more spaces for the swollen spinal cord, is accepted to be beneficial for patients’ long-term functional recovery [5-7]. During the acute period, a great number of SCI patients underwent laminectomy and/or fusion (lam/fusion) [7]. Hardto- treat symptoms, especially pain still exist post-laminectomy. Based on the latest report from Swiss, pain was reported by 68.9% and chronic pain by 73.5% among all the participating SCI patients. The most frequently reported pain location, as reported by 54.6% patients, was the back/spine [8]. Neuropathic pain post SCI remains poorly understood, some researchers attributed it to the nerve lesions [9,10].
Our previous clinical and experimental studies showed evidence of laminectomy leading to failed back surgery syndrome. The failure has been widely accepted to be caused by Epidural Fibrosis (EF). The formatted epidural scar can squeeze the spinal cord and/or nerve root, resulting in radicular pain post laminectomy [11-16]. MRI scans done during our stem cells transplantation studies proved EF in the animal spinal cord injury model post laminectomy [17-19]. We proposed the hypothesis that neuropathic pain can be exacerbated by EF post laminectomy in SCI, because EF can stimulate a repeat occurrence of spine cord compression after surgical repair through the formation of epidural scar tissue. An epidural scar can be readily formed due to the high level of inflammatory activity in the SCI area.
In the present research with SCI rats, we performed macroscopic evaluation, histology, and MR scans. The evidence highly suggested that EF is the unappreciated cause of spinal cord compression post laminectomy in rats with induced SCI. Meanwhile, EF is more easily formed in SCI rats.
Materials and Methods
Animals and group allocation
Adult Sprague-Dawley female rats (n=30) weighted 180-220 g were used in the present study. Rats were randomly divided into three groups with 10 rats in each group: (1) mild injury group (laminectomy and spinal cord impacting, force value=100 kdyn), (2) severe injury group (laminectomy and spinal cord impacting, force value=200 kdyn), (3) control group (laminectomy).
All animal experiments should comply with the ARRIVE guidelines and should be carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments, and were approved by the Ethics Committee of Pirogov Russian National Research Medical University.
Rat model
The rat model was created as previously reported [13,14,18]. Ketamine (50 mg/kg) was intraperitoneally employed, for satisfactory anesthetization. A warm pad was used to maintain the animal’s body temperature during surgery (Braintree Scientific INC, USA). All rats were placed in the prone position and the dorsal fur was shaved. Iodophor (KMN3, Kazan Medical Instruments, Plant Joint Stock Co) was used to sterilize the surgical area. The standard laminectomy was performed at the level of thoracic 9-10 segment (T9-T10).
For the rats in injury groups, the force of 100 kilodynes or 200 kilodynes induced an impact injury to the spinal cord using the PSI-IH Impactor (Precision Systems and Instrumentation LLC, Fairfax, VA). Then the wound was flushed with ice saline, and the wound site was surgically sutured. For the rats in control group, hemostasis with ice saline and surgical suture was performed after standard laminectomy.
Baytril (subcutaneous injection, 2.5 mg/kg/d; Bayer, USA) was used on all the animals. After surgery, the animals in the injury groups received manual bladder expression, twice daily, until the rats gained spontaneous urinary reflection. The control group also received the manual bladder expression to avoid difference.
Behavioral analysis
In order to prove the successful creation of the animal SCI model, Basso, Beattie, and Bresnahan (BBB) locomotor test was performed to evaluate the hindlimb motor behavior in all groups, in a double-blind fashion before injury and weekly post injury [20].
MRI scans
In order to further prove the creation of SCI and EF. MRI scans were done 4 weeks post-operatively. Inhalation anesthesia was performed with the E-Z Anesthesia system (EZ-7000 330, PA, USA), and isoflurane (5DG9621, BAXTER, USA) was used. Using 7 Tesla animal MRI scanners (ClinScan, Bruker BioSpin, USA), MRI scans were performed on the fourth week post-operatively. For sagittal images: T2-weighted imaged in sagittal plane were acquired by Turbo Spin Echo sequence with following parameters: FOV 100 × 49.2 mm, base resolution 256 × 126, TR=3850 ms, TE=42 ms, slice thickness 1 mm, number of acquisition=3, echo train length=9.
Macroscopic assessment of epidural fibrosis
Macroscopic assessment was performed 4 weeks post-operatively in a double-blind fashion. After satisfactory anesthetization, the dorsal fur was re-shaved and the surgical sites were reopened. Epidural scar adhesion was then evaluated based on the Rydell score as following: Grade 0, Epidural scar tissue was not adherent to the dura mater; Grade 1, Epidural scar tissue was adherent to the dura mater, but easily dissected; Grade 2, Epidural scar tissue was adherent to the dura mater, and difficultly dissected without disrupting the dura matter; Grade 3, Epidural scar tissue was firmly adherent to the dura mater, and could not be dissected [13,21].
Hematoxylin-eosin (H and E) staining
Based on our previous reports, H and E staining was performed to evaluate epidural scar formation [12-14]. Rats were deeply anesthetized, then saline and 4% paraformaldehyde (SIGMAALDRICH, USA) in 0.1 M phosphate (SIGMA-ALDRICH, USA) buffer was used for perfusing. The entire T9 and T10 vertebral columns, including the paraspinal muscles and epidural scar tissue, were resected en bloc. The collected samples were then decalcified and dehydrated with Cal-Ex II solution (Thermo Fisher Scientific, USA) for 2 days. After paraffin embedding, 5-μm axial sections of the prepared samples were stained with hematoxylin and eosin. The light microscope (Leica DM500-TR, Germany) was used to evaluate epidural scar adhesion three areas were selected from the center and the margins of each section for the fibroblasts and inflammatory cell count.
Statistical analysis
All statistical analyses were performed by SPSS 17.0 software package (SPSS Inc., Chicago, USA). Results were considered to be statistically significant with p<0.05. All data are presented as mean ± Standard Error of the Mean (SEM), and one-way Analysis of Variance (ANOVA) was employed.
Results
Animal model creation
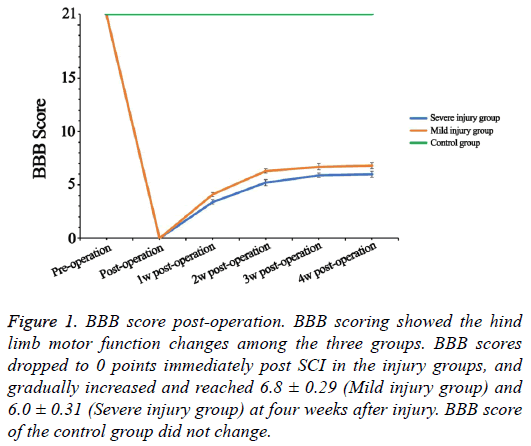
As shown in the Figure 1, the preoperative BBB scores were 21 points in all rats. The rats in the injury groups showed significant loss of hind limbs motor function immediately after SCI, and the BBB scores dropped to 0 points (Figure 1). The BBB score gradually increased and separately reached 6.8 ± 0.29 (Mild injury group) and 6.0 ± 0.31 (Severe injury group) at four weeks after injury. During the four weeks, the BBB score of the control group remained at 21 points.
Figure 1: BBB score post-operation. BBB scoring showed the hind limb motor function changes among the three groups. BBB scores dropped to 0 points immediately post SCI in the injury groups, and gradually increased and reached 6.8 ± 0.29 (Mild injury group) and 6.0 ± 0.31 (Severe injury group) at four weeks after injury. BBB score of the control group did not change.
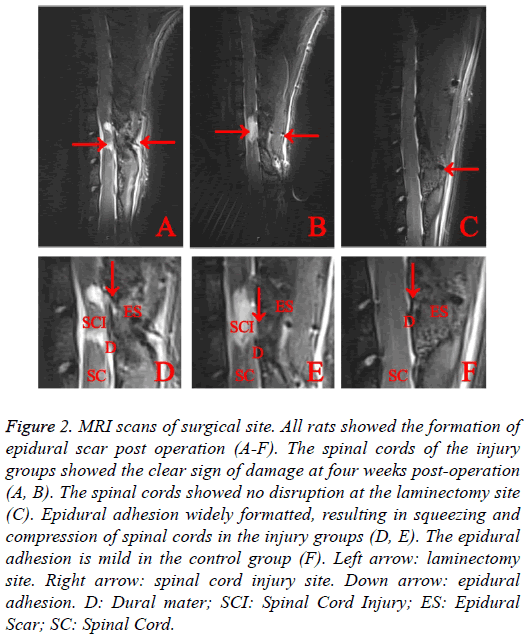
MRI scans suggested the expected spinal cord injury in the experimental groups (Figure 2), while no evidence of SCI was noted, EF was seen in the control group. MRI scans suggested all rats showed the sign of epidural scar formation. Based on the sagittal scans, compared with injury groups which showed severe epidural scar adhesion, control group showed mild epidural scar adhesion. The Epidural Scar (ES) in injury groups was observed to cause severe the spinal cord compression.
Figure 2: MRI scans of surgical site. All rats showed the formation of epidural scar post operation (A-F). The spinal cords of the injury groups showed the clear sign of damage at four weeks post-operation (A, B). The spinal cords showed no disruption at the laminectomy site (C). Epidural adhesion widely formatted, resulting in squeezing and compression of spinal cords in the injury groups (D, E). The epidural adhesion is mild in the control group (F). Left arrow: laminectomy site. Right arrow: spinal cord injury site. Down arrow: epidural adhesion. D: Dural mater; SCI: Spinal Cord Injury; ES: Epidural Scar; SC: Spinal Cord.
Macroscopic examination of epidural fibrosis
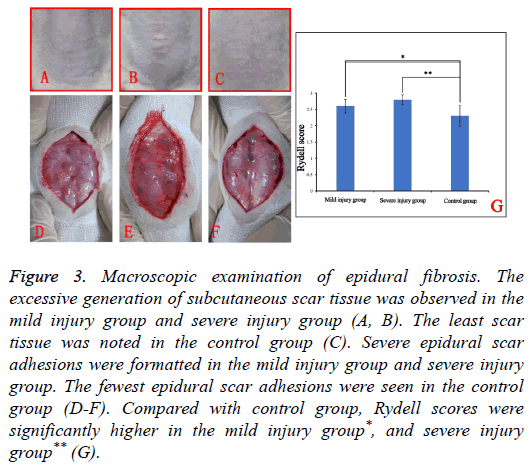
None of the rats expired during the whole process of the study, the rats in injury groups regained spontaneous urination 7-12 d post-operation. Macroscopic examination of the surgical site suggested the excessive generation of subcutaneous scar tissue in mild injury group and severe injury group (Figures 3A and 3B). Compared with injury groups, subcutaneous scar tissue was minimal to absent in the control group.
Figure 3: Macroscopic examination of epidural fibrosis. The excessive generation of subcutaneous scar tissue was observed in the mild injury group and severe injury group (A, B). The least scar tissue was noted in the control group (C). Severe epidural scar adhesions were formatted in the mild injury group and severe injury group. The fewest epidural scar adhesions were seen in the control group (D-F). Compared with control group, Rydell scores were significantly higher in the mild injury group*, and severe injury group** (G).
Upon reopening the surgical sites of the mild injury group and severe injury groups dense, difficult to dissect adhesions were noted. Attempts to dissect these adhesions often caused disruptions of the dura mater with hemorrhages (Figures 3D and 3E).
Among the three groups, the fewest epidural scar adhesions were seen in the control group (Figure 3F). Rydell scoring showed that compared with the control group score (2.30 ± 0.32), the scores of mild injury group (2.60 ± 0.21) and severe injury group (2.80 ± 0.15) were significantly higher, which revealed the least formation of epidural scar formation among three groups (Figure 3G). The excessive formation of epidural scar tissue in the injury groups was observed to compress the spinal cord severely.
Histological analysis
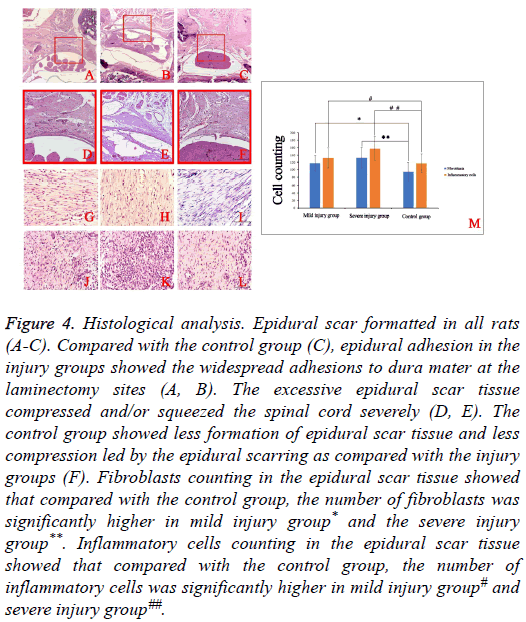
Epidural scar formation was observed in all rats. Compared with control group (Figure 4C), the extent of epidural adhesion in the injury groups showed the widespread adhesions to dura mater at the laminectomy sites (Figures 4A and 4B). In the injury groups (Figures 4D and 4E), the excessive generation of epidural scar tissue resulted in severe compression of the spinal cord. Compared with the injury groups, the control group showed less formation of epidural scar tissue and less compression (Figure 4F).
Figure 4: Histological analysis. Epidural scar formatted in all rats (A-C). Compared with the control group (C), epidural adhesion in the injury groups showed the widespread adhesions to dura mater at the laminectomy sites (A, B). The excessive epidural scar tissue compressed and/or squeezed the spinal cord severely (D, E). The control group showed less formation of epidural scar tissue and less compression led by the epidural scarring as compared with the injury groups (F). Fibroblasts counting in the epidural scar tissue showed that compared with the control group, the number of fibroblasts was significantly higher in mild injury group* and the severe injury group**. Inflammatory cells counting in the epidural scar tissue showed that compared with the control group, the number of inflammatory cells was significantly higher in mild injury group# and severe injury group##.
In order to further demonstrate the difference among three groups. Fibroblasts and inflammatory cells in the epidural scar tissues were counted (Figures 4G-4L). Compared with control group, the numbers of fibroblasts and inflammatory cells in the injury groups were significantly higher (Figure 4M).
Discussion
Epidural fibrosis is the development of the deposition of epidural scar tissue adjacent to the spinal cord and/or nerve roots. Failed Back Surgery Syndrome (FBSS) is characterized by continued pain in the lower posterior trunk and/or lower extremities following laminectomy. It was reported that EF formation contributes to up to 24% of FBSS cases [22]. EF severely limited the efficacy of surgery. Thus, global researchers and physicians have been focusing on the development of an effective strategy to treat the formatted EF and to prevent EF formation.
Although the detailed mechanism of EF formation has not yet been understood, the present study gave one hint. Based on the previous report, chronic SCI results in accelerated atherogenesis through maintaining a low-degree chronic inflammatory state [23]. As our previous reports suggested, aggravating EF led to the increasing expression of some proinflammatory cytokines, such as including interleukin 6, Tumor Necrosis Factor (TNF)-α [11,13,14]. These proinflammatory cytokines can further activate the signaling pathways which are involved in regulating inflammation, such as NF-κB signaling pathway [13]. The low-degree chronic inflammatory state led by SCI, amplified the signaling pathway activation. Fibroblasts proliferation and migration in the surgical site was activated by a number of inflammatory cells.
Based on the previous report, how severe the degree of resulting fibrosis as a sequelae of migration largely depends on the intensity and duration of inflammation [24]. Fibroblasts as the major epidural scar cell source played the central role in epidural scar formation. The formatted epidural scar is capable of compressing and the squeezing of spinal cord/nerve root. We hypothesized to patients with a low-degree chronic inflammatory state pre-laminectomy are at greater risk for EF formation.
Neuropathic pain is accepted to be one of the most troubling adverse sequelae post SCI, which was accompanied by several factors, such as anger, negative thinking, and low mood [25]. Pain is able to completely overwhelm the patients’ existence [26]. The detailed mechanism leading to neuropathic pain remains poorly understood. For a certain proportion of SCI patients spinal cord compression is one of the factors leading to neuropathic pain [6]. Laminectomy is the widely accepted strategy for the decompression of an injured, swollen spinal cord [5]. However, the spinal cord compression may reoccur post laminectomy with EF formation in the chronic SCI patients. Neuropathic pain can be aggravated for the formation of EF in the chronic stage of SCI.
The present study is the first study looking into the correlation between EF and SCI. With laminectomy rats with/without SCI, we demonstrated that EF development can be aggravated by the existence of SCI post laminectomy. This may well explain part of the factors accelerating EF formation. Based on the MRI scans and histological analysis from the present research, the excessive generation of epidural scar caused severe spinal cord/nerve root compression. The spinal cord/nerve root compression could exacerbate neuropathic pain post SCI. With laminectomy rats with mild and severe SCI, the present study revealed that the higher grades of SCI were accompanied by more widespread epidural adhesion, more severe spinal cord compression and the greater neuropathic pain.
We concluded that, in SCI rats, EF is the unappreciated cause of spinal cord compression post laminectomy and epidural scarring can be readily formed. Thus, the present study suggested the necessity of preventing EF for the SCI patients who require surgical intervention in order to diminish the incidence of neuropathic pain post SCI.
More research will be needed to verify our hypothesis that special measures should be taken to prevent EF formation postoperation in patients presenting in an inflammatory state prelaminectomy.
Acknowledgements
This work was supported by the National Science Foundation of China 81602363 and 81270927, and Russian Scientific Foundation 16-15-10432. This work was supported by the Natural Science Foundation of China 81602363 and 81270927, Natural Science Foundation of Tianjin Medical University 2016KYZQ10, and Russian Scientific Foundation 16-15-10432.
References
- Ning GZ, Yu TQ, Feng SQ, Zhou XH, Ban DX. Epidemiology of traumatic spinal cord injury in Tianjin, China. Spinal Cord 2011; 49: 386-390.
- Burns AS, Marino RJ, Flanders AE, Flett H. Clinical diagnosis and prognosis following spinal cord injury. Handbook ClinNeurol 2012; 109: 47-62.
- Ohta K, Fujimura Y, Nakamura M, Watanabe M, Yato Y. Experimental study on MRI evaluation of the course of cervical spinal cord injury. Spinal Cord 1999; 37: 580-584.
- Beers GJ, Raque GH, Wagner GG, Shields CB, Nichols GR, Johnson JR, Meyer JE. MR imaging in acute cervical spine trauma. J Comp Assisted Tomography 1988; 12: 755-761.
- Piazza M, Schuster J. Timing of surgery after spinal cord injury. NeurosurgClin N Am 2017; 28: 31-39.
- Jalan D, Saini N, Zaidi M, Pallottie A, Elkabes S, Heary RF. Effects of early surgical decompression on functional and histological outcomes after severe experimental thoracic spinal cord injury. J Neurosurg Spine 2017; 26: 62-75.
- Boakye M, Patil CG, Santarelli J, Ho C, Tian W, Lad SP. Laminectomy and fusion after spinal cord injury: national inpatient complications and outcomes. J Neurotraum 2008; 25: 173-183.
- Muller R, Brinkhof MW, Arnet U. Prevalence and associated factors of pain in the Swiss spinal cord injury population. Spinal Cord 2016.
- Attal N, Fermanian C, Fermanian J, Lanteri-Minet M, Alchaar H. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain 2008; 138: 343-353.
- Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, Middleton JW, Henderson LA, Siddall PJ. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain 2009; 141: 52-59.
- Zhang C, Feng S, Hu N, Bryukhovetskiy AS, Chekhonin VP. Letter to the editor regarding: Evaluation of topical application and systemic administration of rosuvastatin in preventing epidural fibrosis in rats by Bora Gurer. Spine J Off North Am Spine Soc 2015; 15: 1165-1166.
- Zhang C, Baklaushev VP, Alexandnovich MP, Feng S, Bryukhovetskiy AS. Osteopontin induces the extension of epidural fibrosis into the spinal canal. Pain Physician 2015; 18: 93-95.
- Zhang C, Kong X, Ning G, Liang Z, Qu T, Chen F, Cao D, Wang T, Sharma HS, Feng S. All-trans retinoic acid prevents epidural fibrosis through NF-kappaB signaling pathway in post-laminectomy rats. Neuropharmacol 2014; 79: 275-281.
- Zhang C, Kong X, Liu C, Liang Z, Zhao H, Tong W, Ning G, Shen W, Yao L, Feng S. ERK2 small interfering RNAs prevent epidural fibrosis via the efficient inhibition of collagen expression and inflammation in laminectomy rats. BiochemBiophys Res Commun 2014; 444: 395-400.
- Sun J, Zhang C, Ning G, Li Y, Li Y, Wang P, Feng S. Surgical strategies for ossified ligamentumflavum associated with dural ossification in thoracic spinal stenosis. J ClinNeurosci Off NeurosurgSocAus 2014; 21: 2102-2106.
- Zhang C, Kong X, Zhou H, Liu C, Zhao X, Zhou X, Su Y, Sharma HS, Feng S. An experimental novel study: Angelica sinensis prevents epidural fibrosis in laminectomy rats via downregulation of hydroxyproline, IL-6, and TGF- beta 1. Evi Bas Compl Alt Med 2013; 2013: 291814.
- Zhang C, Feng S, Hu N, Douglas P, Chekhonin VP. Letter to the editor regarding Local versus distal transplantation of human neural stem cells following chronic spinal cord injury by Cheng. Spine J Off N Am Spine Soc 2016; 16: 792-793.
- Zhang C, Morozova AY, Abakumov MA, Gubsky IL, Douglas P, Feng S, Bryukhovetskiy AS, Chekhonin VP. Precise delivery into chronic spinal cord injury syringomyelic cysts with magnetic nanoparticles MRI visualization. Med SciInt Med J ExpClin Res 2015; 21: 3179-3185.
- Ning G, Tang L, Wu Q, Li Y, Li Y, Zhang C, Feng S. Human umbilical cord blood stem cells for spinal cord injury: early transplantation results in better local angiogenesis. Regen Med 2013; 8: 271-281.
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotraum 1995; 12: 1-21.
- Rydell N. Decreased granulation tissue reaction after installment of hyaluronic acid. ActaOrthopaedicaScandinavica 1970; 41: 307-311.
- Burton CV, Kirkaldy-Willis WH, Yong-Hing K, Heithoff KB. Causes of failure of surgery on the lumbar spine. ClinOrthopRelat Res 1981; 191-199.
- Wang TD, Wang YH, Huang TS, Su TC, Pan SL, Chen SY. Circulating levels of markers of inflammation and endothelial activation are increased in men with chronic spinal cord injury. J Formosan Med Assoc Taiwan Yi Zhi 2007; 106: 919-928.
- Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease - Current knowledge and future perspectives. J Crohns Colitis 2008; 2: 279-290.
- Dugan EA, Sagen J. An intensive locomotor training paradigm improves neuropathic pain following spinal cord compression injury in rats. J Neurotraum 2015; 32: 622-632.
- Buscemi V, Cassidy E, Kilbride C, Reynolds FA. A qualitative exploration of living with chronic neuropathic pain after spinal cord injury: an Italian perspective. Disab Rehab 2017; 1-10.