Research Article - Biomedical Research (2017) Volume 28, Issue 13
EphA2, EphrinAl expression in breast cancer and its relationship with clinical pathological study
Siyuan Bian, Bensi Zhang*, Gui Yang, Qiandai Song and Yanjiao Li
Department of Human Anatomy, College of Medical Sciences, Dali University, Dali 671000, PR China
Accepted on May 29, 2017
Abstract
Objective: To investigate the expression of EphA2 and EphrinAl in breast cancer from Yunnan Dali Bai, Han and other minority women and to investigate their relationship with clinicopathological parameters, ie., the tumor size, pathological type, lymph node metastasis, age, TNM staging, and the histological grade.
Methods: Using immunohistochemical SP method and in situ hybridization to detect the expression aiming at the protein and mRNA of EphA2 receptor and its ligand EphrinAl in the breast cancer, including 129 cases of invasive ductal carcinoma, 8 cases of intraductal carcinoma and 40 cases of normal breast tissues.
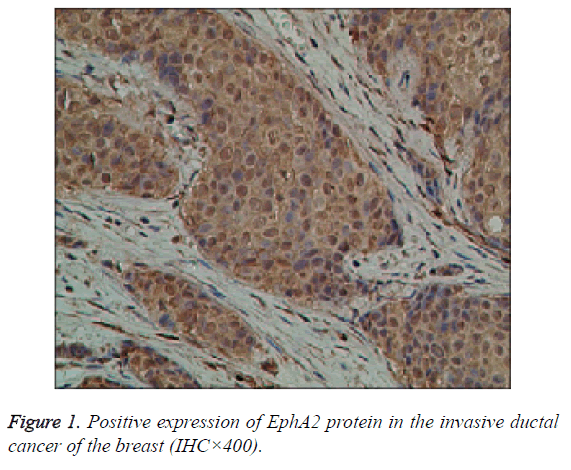
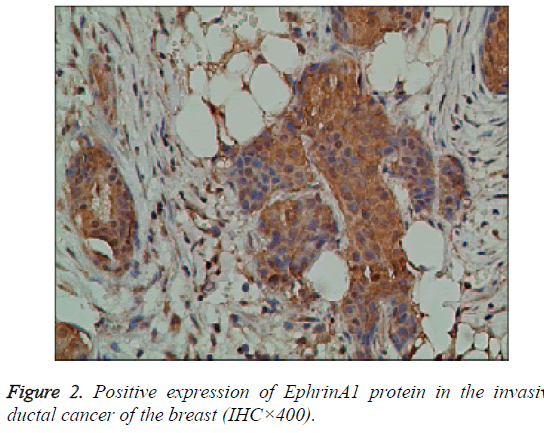
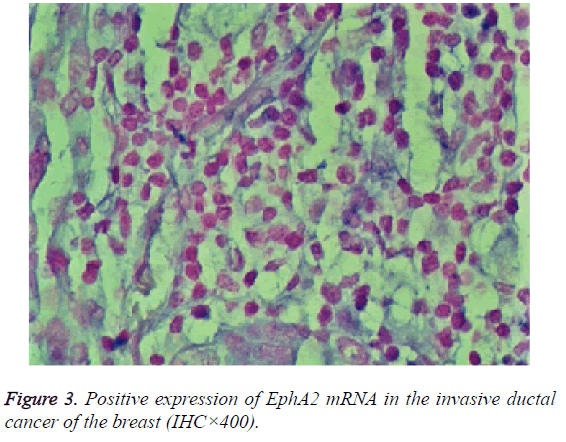
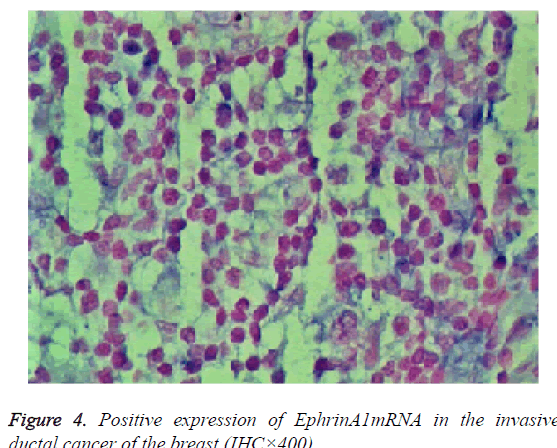
Results: The immunohistochemistry showed that the positive expression rate of EphA2 was 89% and that of EphrinAl was 94% in 129 cases of invasive ductal carcinoma tissues, while in 8 cases of intraductal carcinoma the positive rate of EphA2 and of EphrinAl was 63% and 75%, respectively. In 40 cases of normal tissue EphA2 positive expression rate was 3%, and the EphrinAl positive rate was 2%. There is a significant difference between the positive expression rate of EphA2 and that of EphrinAl (P<0.05). In addition, in situ hybridization showed that the positive expression rate of EphA2 was 73% and that of EphrinAl was 80% in 129 cases of invasive ductal carcinoma tissues. In 8 cases of intraductal carcinoma the positive rate of EphA2 and of EphrinAl was 37% and 50%, respectively. In 40 cases of normal tissue EphA2 positive expression rate was 1%, and EphrinAl positive rate was 2%. There is a significant difference between the positive expression rate of EphA2 and that of EphrinAl (P<0.05). The positive expression rates of EphA2 and EphrinAl have no significant correlation with the patient age and TNM staging (P>0.05), but are correlated statistically with the pathological type, tumer size, lymph node metastasis and histological grade (P<0.05). There is a significant difference between the positive expression rate of the EphA2 protein and that of EphA2 mRNA, and between that of EphrinA1 protein and its mRNA (P<0.05).
Conclusion: EphA2 and EphrinAl show very strong expression in breast cancer tissue, and may play an important role in the pathophysiological research and in the diagnosis and treatment of breast cancer.
Keywords
EphrinAl, EphA2, Breast cancer, Immunohistochemistry, In situ hybridization
Introduction
As the highest prevalence of gynaecological malignanciesthe morbidity of breast cancer is persistent high growth and keeps up the morbidity annually. Breast cancer is a typical disease as malignant tumors for female patients in a modern city and it has been posing a great threat to human health and quality of life. Eph family proteins include Eph receptor and Ephrin ligand. In recent years, scientific studies show that EphA2 is not only expressed in a high percentage of lung cancer, cervical cancer, and other diseases, but also strongly expressed in the gallbladder, pancreas and other organs. In addition, a B2 receptor in the family is highly expressed in breast cancer. On the basis of the data that 137 cases of breast cancer and 40 cases of adjacent normal tissues diagnosed by the Affiliated Hospital of Dali University, adding in situ hybridization under the conventional immunohistochemistry premise, the expression of EphA2 and EphrinAl in breast cancer tissues and its relation with the clinocopathological factors are studied through comparison and statistical analysis.
Materials and Methods
General information
This paper selects 137 paraffin-embedded tissues which diagnosed as breast cancer and 40 cases of normal breast tissues by pathological examination between 2013 and 2015 in Dali University affiliated hospital.
Main reagents
The EphA2 and EphrinAl Rabbit Anti-Human Polyclonal Antibody made, ready-to-use Immunohistochemical Ultrasensitive TM SP Box, In situ hybridization experiment box III (alkaline phosphatase) by the Agilent Technologies biological company were purchased in Nanjing Nuowei Chan biological company; Digoxigenin- labelled the EphA2 and EphrinAl cDNA probe were purchased in Changsha Axybio Biotechnology Company Ltd.
Methods
Immunohistochemical testing and result: The routine immunohistochemistry stain was used and replaced primary antibodies with PBS that through using as a negative control, a section who was known as positive used as positive control.
Result: The EphA2 and EphrinAl protein’s positive reaction in the cell that pulp and membrane are shown a brown or tan. By selecting randomly five visions under a high power microscope from every slice, and counting at least five hundred cells, according to staining positive cells account for the percentage of the total number of tumor cells to decide (+) and (-). For EphA2, pulp and membrane in the cell must show a brown or tan, the every five visions >20% is true positive staining, others is negative; For EphrinAl, the every five visions >10% is true positive staining, or negative hybridization in situ.
Hybridization in situ Testing and Results: The paraffinembedded tissues remove paraffin with toluene-absolute alcohol, then through 70% ethanol, 50% ethanol transit to water, reacting in 3% H2O2 at normal temperature for 10 m, washing twice. Exposing the mRNA nucleic acid fragment, add 40 ml 3% pepsin, reacting in 37°C for 15 m; Washing thrice in 0.5 mTBS for 5 m, washing once in distilled water. Pre-hybridization: drop 40 ml pre-hybridization solution in every slice, react in 37°C-40°C for 2-4 h, remove useless liquidno need to wash.
Hybridization: Use digoxigenin-labelled cdna probe, drop about 40 ml hybridization solution, use professional cover slip, put in 56°C oven for 90 m, then put in 37°C incubator hybridize overnight.
Post-hybridization washing: Remove cover slip, in 30-37°C water temperature washing twice in 2×SSC for 5 m×2; washing once in 0.5×SSC for 15 m; washing twice in 0.2SSC for 15 m. Dropping confining liquid: in 37°C for 20 m, get rid of excess liquid, drop labelled digoxigenin: use 0.5 mTBS to dilute in the proportion of 1200, add 40 ml to every slices, in 37°C for 60 m, washing in 0.5 mTBS four times for 5-10 m. BCIP/NBT Coloration: 20xBCIP use 0.01 m spec of TBS solution to dilute in the proportion of 120, mixing, full reaction in 30-37°C, general for 30-60 m. Sufficient washingalcohol dehydratexylene process, finally sealing sheet.
Result: The positive sign of the EphA2 and EphrinAl mRNA that pulp in the cell are shown a purple, the criteria were similar to immunohistochemistry.
Statistical analysis
Using SPSS 16.0 version for data processing, use the X2 test to compare the positive rate of samples and the correlation analysis, if P<0.05, the difference has statistical significantly.
Results
The reaction of the EphA2 and EphrinA1 protein in the Breast cancer and the control group
The EphA2 and EphrinAl protein in cancer group, showed strongly positive expression; in the control group, they showed weak positive expression; the positive expression of the two proteins is mainly in the plasma and vascular endothelial cells of the pulp and membrane (Figures 1 and 2).
The Expression of EphA2 and EphrinA1 in Breast Cancer and Its Correlation with Pathological Factors
The positive expression of EphA2 and EphrinAl protein was not related to age and TNM staging, but correlated with pathological type, lymph node metastasis, tumor size and histological grade (P<0.05) (Tables 1 and 2).
| EphrinA1 | ||||||
| N | + | - | c2 | P | ||
| Year | ||||||
| ≤45 | 52 | 48 | 4 | 0.019 | 0.89 | |
| >45 | 85 | 79 | 6 | |||
| The types | ||||||
| Intraductal carcinoma | 8 | 6 | 2 | 3.934 | 0.047 | |
| Invasive carcinoma | 129 | 121 | 8 | |||
| Size | ||||||
| <5 cm | 95 | 92 | 3 | 7.0855 | 0.005 | |
| ≥5 cm | 42 | 35 | 7 | |||
| TNM staging | ||||||
| I | 26 | 23 | 2 | 2.679 | 0.444 | |
| II | 69 | 65 | 4 | |||
| III | 37 | 36 | 1 | |||
| IV | 5 | 4 | 1 | |||
| Lymphatic metastasis | ||||||
| + | 71 | 69 | 2 | 4.376 | 0.036 | |
| - | 66 | 58 | 8 | |||
| Histological grade | ||||||
| I | 3 | 1 | 2 | 16.127 | 0.0003 | |
| II | 74 | 69 | 5 | |||
| III | 60 | 57 | 3 | |||
Table 1. EphrinA1 protein expression in breast cancer (N=137).
| EphA2 | |||||
| N | + | - | c2 | P | |
| Year | |||||
| ≤45 | 52 | 45 | 7 | 0.085 | 0.77 |
| >45 | 85 | 75 | 10 | ||
| The types | |||||
| Intraductal carcinoma | 8 | 5 | 3 | 4.921 | 0.027 |
| Invasive carcinoma |
129 | 115 | 14 | ||
| Size | |||||
| <5 cm | 95 | 89 | 6 | 10.584 | 0.001 |
| ≥5 cm | 42 | 31 | 11 | ||
| TNM staging | |||||
| I | 26 | 21 | 5 | 5.57 | 0.134 |
| II | 69 | 62 | 7 | ||
| III | 37 | 34 | 3 | ||
| IV | 5 | 3 | 2 | ||
| Lymphatic metastasis | |||||
| + | 71 | 57 | 14 | 7.245 | 0.007 |
| - | 66 | 63 | 3 | ||
| Histological grade |
|||||
| I | 3 | 2 | 1 | 8.641 | 0.013 |
| II | 74 | 60 | 14 | ||
| III | 60 | 58 | 2 | ||
Table 2. EphA2 protein expression in breast cancer (N=137).
The contact of EphA2 and EphrinAl protein positive expression in breast cancer
The EphA2 and EphrinAl protein positive expression basically both in similar areas and vascular endothelial cells, the location is basically the same. Statistical analysis, EphA2 and EphrinAl expression are related (P<0.05) (Table 3).
| EphA2 | |||||
| + | - | c2 | P | ||
| EphrinAl | + | 114 | 13 | 7.556 | 0.006 |
| - | 6 | 4 | |||
Table 3. The comparison of EphA2 and EphrinA1 protein expression in breast cancer.
EphA2, EphrinAl mRNA in breast cancer and the control group
The expression of EphA2 and EphrinAl mRNA was strongly positive in the cancer group, and the expression of EphA2 and EphrinAl mRNA was very positive in the control group; Both mRNAs were predominantly expressed in the plasma of the tumor and vascular endothelial cells (Figures 3 and 4).
EphA2, EphrinAl mRNA expression in breast cancer tissue and the related pathological factors
The positive rate of EphA2 and EphrinAl mRNA was not associated with age and TNM stage (P>0.05), but there was correlation with pathological type, lymph node metastasis, tumor size and histological grade (P<0.05) (Tables 4 and 5).
| EphA2 | |||||
| N | +(%) | -(%) | c2 | P | |
| Year | |||||
| ≤45 | 52 | 37 | 15 | 0.006 | 0.939 |
| >45 | 85 | 61 | 24 | ||
| The types | |||||
| Intraductal carcinoma | 8 | 3 | 5 | 4.832 | 0.028 |
| Invasive carcinoma | 129 | 95 | 34 | ||
| Size | |||||
| <5 cm | 95 | 73 | 22 | 4.29 | 0.038 |
| ≥5 cm | 42 | 25 | 17 | ||
| TNM staging | |||||
| I | 26 | 23 | 3 | 6.517 | 0.089 |
| II | 69 | 48 | 21 | ||
| III | 37 | 25 | 12 | ||
| IV | 5 | 2 | 3 | ||
| Lymphatic metastasis | |||||
| + | 71 | 57 | 14 | 5.54 | 0.019 |
| - | 66 | 41 | 25 | ||
| Histological grade | |||||
| I | 3 | 0 | 3 | ||
| II | 74 | 54 | 20 | 7.709 | 0.021 |
| III | 60 | 44 | 16 | ||
Table 4. EphA2 mRNA expression in breast cancer (N=137).
| EphrinA1 | |||||
| N | +(%) | -(%) | c2 | P | |
| Year | |||||
| ≤45 | 52 | 43 | 9 | 0.748 | 0.387 |
| >45 | 85 | 65 | 20 | ||
| The types | |||||
| Intraductal carcinoma | 8 | 4 | 4 | 4.232 | 0.04 |
| Invasive carcinoma | 129 | 104 | 25 | ||
| Size | |||||
| <5 cm | 95 | 86 | 10 | 21.344 | 0 |
| ≥5 cm | 42 | 23 | 19 | ||
| TNM staging | |||||
| I | 26 | 18 | 8 | 5.117 | 0.163 |
| II | 69 | 54 | 15 | ||
| III | 37 | 34 | 4 | ||
| IV | 5 | 3 | 2 | ||
| Lymphatic metastasis | |||||
| + | 71 | 62 | 9 | 6.369 | 0.012 |
| - | 66 | 46 | 20 | ||
| Histological grade | |||||
| I | 3 | 1 | 2 | ||
| II | 74 | 55 | 19 | 6.83 | 0.033 |
| III | 60 | 52 | 8 | ||
Table 5. EphrinA1mRNA expression in breast cancer (N=137).
The contact of EphA2 and EphrinA1 mRNA positive expression in breast cancer
EphA2 and EphrinAl mRNA expression in a similar region and vascular endothelial cells, the location is basically the same. Statistical analysis, EphA2 and EphrinAl expression are related (P<0.05) (Table 6).
| EphA2 | |||||
| + | - | c2 | P | ||
| EphrinAl | + | 94 | 14 | 60.228 | 0 |
| - | 4 | 25 | |||
Table 6. EphA2 in breast cancer and EphrinA1 mRNA expression.
Discussion
Eph family proteins include Eph receptor and Ephrin ligand. At first, the study shows that its effect maybe control cell movement that shrinks the skeleton of cells and increase the viscosity between cells which to move them together [1]. The regulation process of Eph receptors is broadly divided into several parts, such as embryogenesis, the direction of cell axon movement and vascular regeneration and so on [2]. The Ephrin ligand in the transmission of information between cells, the synergy between each other is significant, at the same time adjust the adhesion between endothelial intercellular and liquidity [2]. In the modern society, the study of how EphA2 and EphrinAl play a role in breast cancer is sustained in early stages, many reaction mechanisms are unclear. The change of EphA2 and EphrinAl’s microenvironment is one of the main factors to make the high expression in breast cancer tissues [3]. When E-cadherin secretion decreased which in the breast tissue, the viscosity of cells and cells will be reduced, the interaction of each other will also reduce. Therefore, it interrupts the normal relation between EphA2 and EphrinAl ligand, leading to the EphA2 and EphrinAl stocking in the breast cancer. Meanwhile, due to the viscidity of EphA2 and Adjacent ligand descent, both are gradually away, this condition named losing phosphorylation, it can interrupt the reaction of EphA2 and downstream signal transduction proteins, make EphA2 cannot be degraded normally and resulting in a large accumulation of EphA2.
In the experiment which includes 137 cases of carcinoma tissue, the EphrinAl and EphA2 protein expression positive cases were 114 cases, the negative were 4 cases, they all were highly expressed in breast cancer. Meanwhile, the EphrinAl and EphA2 mRNA co-positive expression were 94 cases, the co-negative was 25 cases, and they all were highly expressed in breast cancer. In the clinical pathological factors, both of the expression rates was not associated with age and TNM stage, but with the development of tumors, the severity of infiltration and a higher histological grade, the positive rate of EphrinAl and EphA2 will be higher, it was positively correlated. In the research, EphA2 proteins and mRNA positive expressions were 87% and 71% respectively. EphrinAl were 92% and 78%. The results of EphA2 protein expression were almost the same to cancer in other tissue regions such as breast, esophageal, colon, lung, prostate, ovary, cervix and melanoma [4-14]. On the test method, the positive expression of EphA2 and EphrinA1 in cancer tissues was significantly higher than that in normal breast tissues by immunohistochemistry and in situ hybridization, the positive expression of EphA2 and EphrinA1 is positive correlation with tumors size, the depth of the infiltration, whether lymph node metastasis, histological grade, and other clinicopathological factors. In statistical analysis, the expression of EphA2 and EphrinAl in the invasive group, lymph node metastasis, tumor size and histological grading have statistically significant (P<0.05).
These results confirmed that EphA2 and EphrinAl have the high expression in breast cancer; both of high expressions maybe promise the severity of breast cancer and prognosis.
Lots of experiments have shown that the progress of the tumor and Eph receptor and Ephrin ligand have closely linked, Zelinski et al. proved by experiments, the over-expression of EphA2 can make MCF-10A mammary epithelial cells to be vicious transformation rapidly, reducing the adhesion between tumor cells, leading to cell cannot be a good connection, promoting the growth and metastasis of tumor [15,16] Easy et al. found that the positive expression rate of EphA2 and EphrinAl were positively correlated with the severity of tumor, the positive rate of advanced tumor is very high, meanwhile it also related to the survival state. Brian and other studies have shown that EphA2 has high expression in breast cancer, and its upto tumor progression. Herron’s studies for a large number of cases of Renal Cell Carcinoma (RCC) suggest that high expression of EphA2 RCC tumor invasion deeper, higher clinical stage, and the over-expression of EphA2 for tumor recurrence interval and mortality predictors is significant. The experimental analysis that it maybe make cell adhesion decreased, cannot have a synergistic effect, then block the combination of EphA2 and ligand and its degradation, make it cannot reduce normal physiology functions. The results of this study demonstrate that EphA2 and EphrinAl are closely related to breast cancer, which provides further experimental evidence for the above theoretical studies.
To conclude, the high expression of EphA2 and EphrinAl in breast cancer is closely related to the malignancy of breast cancer, the expression of EphA2 and EphrinAl on the clinic is likely to be a new marker for the diagnosis and treatment of breast cancer. EphA2 and EphrinAl would be a new targeted therapeutic target for breast cancer; it may lead to earlier detection of breast cancer and earlier treatment [17]. Our study of EphA2 and EphrinAl ligand will likely to be a more accurate approach for the treatment of breast cancer [18].
Funding
The study received financial support from the National Natural Science Foundation of China (No. 81460465).
References
- Tsouko E, Wang J, Frigo DE. miR-200a inhibits migration of triple-negative breast cancer cells through direct repression of the EPHA2 oncogene. Carcinogenesis 2015; 36: 1051-1060.
- Congxin Q, Xiaojun G, Quanyuan L. EphA2, SIAH2 in breast cancer tissue, the expression of beta-catenin and malignancy associated. Clin Experi Med 2016; 15: 844-847.
- Suetterlin P, Marler KM, Drescher U. Axonal ephrinA/EphA interactions and the emergence of order in topographic projections. Semin Cell Dev Biol, 2012, 23: 1-6.
- Yang X, Cheng L, Dai S. Increased EphA/EphrinA expression in hippocampus of pilocarpine treated mouse. Epilepsy Res 2013; 105: 20-29.
- Kazi AA, Gilani RA, Schech AJ. Nonhypoxic regulation and role of hypoxia-inducible factor 1 in aromatase inhibitor resistant breast cancer. Breast Cancer Res 2014; 16: R15.
- Giannoni E, Taddei ML, Parri M. EphA2-mediated mesenchymal-amoeboid transition induced by endothelial progenitor cells enhances metastatic spread due to cancer-associated fibroblasts. J Mol Med 2013; 91: 36-42.
- Philipp S, Uwe D. Target-independent EphrinA/EphA-mediated axon-axon repulsion as a novel element in retinocollicular mapping. Neuron 2014; 84: 740-752.
- Barquilla A, Pasquale EB. Eph receptors and Ephrins: therapeutic opportunities. Annu Rev Pharmcol Toxicol 2015; 55: 465-87.
- Shao Z, Zhu,F, Song K. EphA2/EphrinA1 mRNA expression and protein production in adenoid cystic carcinoma of salivary gland. J Oral Maxillofacial Surgery 2013; 71: 10-14.
- Yin H, Lu C, Tang Y. Enhanced expression of EphrinB1 is associated with lymph node metastasis and poor prognosis in breast cancer. Cancer Biomarkers 2013; 13: 261-267.
- Boissier P, Chen J, Huynh-Do U. Epha2 signalling following endocytosis: Role of tiam1. Traffic 2013; 14: 71-75.
- Yao N, Ren K, Jiang C. Combretastatin A4 phosphate treatment induces vasculogenic mimicry formation of W256 breast carcinoma tumor in vitro and in vivo. Tumour Biol 2015; 36: 8499-8510.
- Yang P, Yuan W, He J. Overexpression of EphA2, MMP-9 and MVD-CD34 in hepatocellular carcinoma: implications for tumor progression and prognosis. Hepatol Res 2009; 39: 1169-1177.
- Hsieh SC, Kuo SN, Zheng YH. The E3 ubiquitin ligase S1AH2 is a pm survival factor overexpressed in oral cancer. Anticancer Res 2013; 33: 4965-4973.
- Kazi AA, Gilani RA, Schech AJ. Non-hypoxic regulation and role of hypoxia-inducible factor 1 in aromatase inhibitor resistant breast cancer. Breast Cancer Res 2015; 16: 1-18.
- Yanyuan W, Hezla M, Ram C, Ishrat A, Sheila C, Dennis S, Jaydutt VV. Clinical significance of Akt and HER2/neu over-expression in African-American and Latina women with breast cancer. Breast cancer Res 2016; 10: 98-99.
- Yao N, Ren K, Jiang C. Combretastatin A4 phosphate treatment induces vasculogenic mimicry formation of W256 breast carcinoma tumor in vitro and in vivo. Tumour Biol 2015; 36: 8499 -8510.
- Rosa M, Han HS, Ismail KR. Beta-catenin expression patterns in matched pre and post-neoadjuvant chemotherapy-resistant breast cancer. Ann Clin Lab Sci 2015; 45: 10-16.