Research Article - Biomedical Research (2017) Volume 28, Issue 6
Environmental surfaces in healthcare setting: a great potential risk of pathogens transmission
Bouchra Oumokhtar1*, Abdelhakim El Ouali Lalami2, Nadia Benaicha3, Btissam Arhoune1 and Wafaa Bono4
1Laboratory of Microbiology, Faculty of Medicine and Pharmacy, Sidi Mohamed BenAbdallah University, Morocco
2Higher Institute of Nursing Professions and Health Techniques Fez (Annexe Meknes), Regional Health Directorate, EL Ghassani Hospital, Morocco
3Laboratory of Epidemiology, Clinical Research and Health Community, Faculty of Medicine and Pharmacy, Sidi Mohamed BenAbdallah University, Morocco
4Internal Medicine Ward, Hassan II University Hospital, Faculty of Medicine and Pharmacy, Sidi Mohamed BenAbdallah University, Morocco
- *Corresponding Author:
- Oumokhtar Bouchra
Laboratory of Microbiology
Faculty of Medicine and Pharmacy
Sidi Mohamed BenAbdallah University, Morocco
Accepted date: October 15, 2016
Abstract
The aim of our study is to estimate bacterial contamination of the surfaces surrounding hospitalized patients. This study was achieved from March to June 2014, in an internal medicine ward at the Hospital University Hassan II in Fez. By using the swab technique, the samples were taken from different surfaces surrounding hospitalized patients. Bacterial analysis was performed according to conventional culture and identification techniques. Antimicrobial susceptibility was determined for each isolate by the disk diffusion method. During the study period, 112 samples were collected from different sites in 10 rooms. The bedrails are the most contaminated sites (100%), followed by bedsides tables (60%). Toilets door knobsand room knobs come in third place (50%). We identified 200 isolates of bacteria: Coagulase Negative Staphylococci, Staphylococcus aureus, Enterobacter sp., Pantoea sp., Klebsiella sp., Escherichia coli, Pseudomonas sp., Stenotrophomonas sp. and Enterococcus sp. Different antibiotic resistance profiles were registered for isolated bacteria of which 1 isolate of Enterobacter aerogenes and 1 E. coli are ESBL. We report in this study that hospital environment is contaminated by a variety of pathogenic and opportunist, resistant and sensitive bacteria isolated from many surfaces sites of patients rooms. Hospital must implement evidence-based infection prevention measures that will reduce the risk of transmission of pathogens via contaminated hospital surfaces and medical equipment.
Keywords
Hospital environment, Bacterial contamination, Nosocomial transmission, Fez, Morocco.
Introduction
Health-care associated infections HAIs are among the major causes of death and the increasing morbidity for hospitalized patients. The issue of the risk of patient infection through contaminated hard surfaces in hospital rooms has been widely discussed [1,2]. Environment represents the external source of HAI and includes air, food, water, surfaces surrounding the patient and medical devices. Patient environment are frequently contaminated by nosocomial pathogens, and are considered as a reservoir for pathogens transmission directly through patient contact with the environment or indirectly through contamination of health care workers' hands and gloves [3]. The contaminated surfaces contribute to the epidemic and endemic transmission of several pathogens including Gram-positive pathogens like Clostridium difficile, Methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-resistant Enterococcus (VRE), and Gram-negative pathogens (Pseudomonas, Klebsiella and Acinetobacter sp.) as well as some viruses [3]. Furthermore, several studies have reported that contaminated surfaces in hospital are a wellrecognized cause of common-source outbreaks of infection [1,4]. Moreover, hospitalization in a room in which the previous patient had been colonized or infected with these pathogens has been shown to be a risk factor for colonization or infection with the same pathogen for the next occupant admitted to the room [5,6]. Thus, microbial evaluation of surfaces is useful in monitoring the effectiveness of the cleaning and disinfecting practices. So, the aim of this study was to estimate bacterial contamination of the inanimate environment surrounding patients, and also to examine the presence of specific nosocomial multi-resistant pathogens which may be the cause of nosocomial infection. This information will be needed for the control program and for the improvement pathogenic control practices.
Materials and Methods
Study design
This study was performed in HASSAN II University Hospital UH, a 880-bed tertiary care center located in Fez city. The study was carried out in response to demand from head of internal medicine ward, to evaluate the efficacy of routine cleaning and disinfection practices. However, neither cleaning nor ward staffs were informed about the environmental sampling, which was performed at random intervals during the day working. This study was undertaken during non-outbreak settings. Cleaning and disinfecting practices in the service are conducted as a routine activity by environmental services workers, using a quaternary amine based product or bleach, without any specific protocols. The cleaners have no training for what they are supposed to be doing. Internal medicine ward is consisting of 10 rooms housing about 24 beds; it is a specific unit which receives particularly immuno-compromised patients with complex medical problems of affecting blood, cancers and infections (AIDS). This makes these patients particularly susceptible to acquire nosocomial infection.
Samples collection and bacterial analysis
Swab technique is the method that we used to collect the samples from the surfaces of patients’ rooms. During four months (March-June 2014) and in the absence of a known outbreak, the samples were taken weekly from environment surfaces surrounding hospitalized patients in all rooms of service. The following surfaces were chosen for sampling: bedrails, bedside tables, toilets door knobs, room's door knobs, electricity buttons, cupboards knobs and chemotherapy chairs arm.
Sterile cotton swabs pre-moistened in a buffer solution were used for sampling surfaces. At each site, an area of approximately 10 cm2 was swabbed in two directions at right angles to each other in a close zigzag pattern while rotating the swab during sampling to ensure that the entire surface of the swab was used according to the guideline (Norme ISO/DIS 14698-1, 1999). Then, the swab was wetted in tubes containing Brain Heart Infusion Broth (BHI) that encourages the growth of microorganisms. The collected samples are transported immediately to the laboratory, and incubated for 24 to 48 h at 37°C. Selective and specific media were used: Mannitol salt agar for Staphylococci, Eosin-Methylen Blue Agar for Enterobacteriaciae, Cetremide Agar for Pseudomonas sp. Representative bacteria strains were selected on the basis of colony morphology, Gram staining, oxydase and/or catalase tests. Full identification of the bacteria was made by conventional biochemical techniques and confirmed by Galerie API 20E®, API 20NE® and Api Staph® tests according to the manufacturer’s instructions (BioMérieux, France).
Antimicrobial susceptibility testing
Antimicrobial susceptibility was determined for each isolate by the disk diffusion method on Mueller-Hinton (MH) agar plates (Bio-Rad, Marnes-la-Coquette, France). The tested antibiotics were chosen depending on species identified according to the Committee of Antimicrobial Testing, French Society of Microbiology (CA-SFM, 2013).
Results
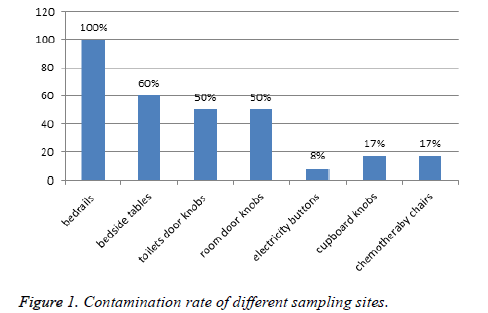
A total of 112 samples were collected from different sites in 10 rooms during the study period. These samples are distributed in the following surfaces sites of patients rooms: bedrails, bedside tables, toilets door knobs, room’s door knobs, electricity buttons, cupboards knobs and chemotherapy chairs arm. These chairs were reserved for AIDS patients. All the sampled patient rooms were positive for at least 1 gender of bacteria at one or more environmental sites. The bedrails are the most sites contaminated (100%), followed by bedsides tables (60%), toilets door knobs and room’s door knobs in third place (50%) (Figure 1).
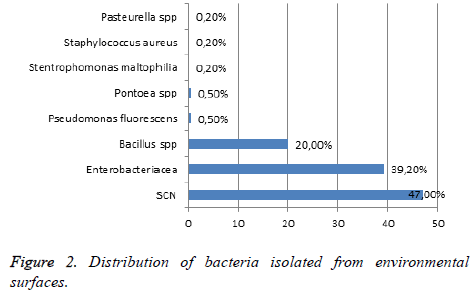
From 112 collected surfaces samples, we identified 200 isolates of bacteria and 38 isolates have not been identified despite our effort. CNS were the large types of identified bacteria 94 (47%), 30% of them were taken from bedrails, 19% from bedside tables and room’s doors knobs, 21% from toilets doors knobs, 7% from chemotherapy chairs arm, 2% from cupboard knobs and 2% from electricity buttons. Four isolates of S. aureus were identified, 2 strains on bedrails and 2 on room’s doors knobs. Enterobacteriaceae were present on surfaces analyzed at 39.2%: 18 isolates of Enterobacter sp. (9 strains of E. aerogenes, 8 E. cloacae and 1 E. amnigenus), 10 isolates of Pantoea agglomerans, 10 Klebsiella ozaneae, 2 Ewingella sp. and 2 E. coli. All these species were regularly taken from bed rails and bed side tables (40 to 45%) except for Enterobacter species which were isolated from cupboard knobs (33%) and toilet door knobs (11%). Whereas E. coli and Ewingella sp. were isolated exclusively from toilet door knobs. Furthermore, 10 strains of Pseudomonas fluorescence and 4 Stenotrophomonas maltophilia were taken mainly from bed rails and bed side tables (40%), from toilets and doors knobs (20%), while 50% of Pasteurella sp. (4 strains) were isolated from bedside table and 50% from cupboard knobs. Other species are found in this study: 40 isolates of Bacillus sp. recovered from the all types of the analyzed surfaces: bed rails (40%), on room’s doors knobs and on bedside tables (15% for each), on electricity buttons and on toilets doors knobs (10% for each), on cupboard knobs and on chemotherapy chairs (5% for each). Also, 2 Enterococcus sp was identified from bedrails, and the two isolates are Vancomycin resistant VRE. The study of resistance profile of SCN isolated showed that 85% of bacteria were resistant at least to 1 antibiotic; 71% were resistant to penicillin and erythromycin, 57% were resistant to methicillin and fusidic acid, and 14% were resistant to rifampicin. No resistance of vancomycin and teicoplanin was observed. Concerning S. aureus, two strains of S. aureus were identified as MRSA and all isolates were resistant to penicillin. We noted also a resistance of 50% to erythromycin and of 50% to gentamycin. All isolates of Pseudomonas were resistant to ticarcilline but are sensitive to the other tested antibiotic. Regarding Enterobacteriaceae strains, different antibiotic resistance profiles were registered. One isolate of Enterobacter aerogenes and 1 E. coli are producing Extended Spectrum Beta-lactamase ESBL. These isolates are also resistant to quinolone and fluoroquinolone, gentamycin, tobramycin and trimethoprim-sulfamethaxazole (Figure 2).
Discussion
The meaning of environmental cleaning for reducing microbial contamination of surfaces and subsequent risk for HAIs has been reported extensively [7-9]. However, the efficacy of traditional cleaning methods to remove surface contamination is under debate [10]. The cleaning process itself is subject to debate over frequencies, methods, equipment, benchmarks, monitoring, and standards for surface cleanliness [11]. This study was undertaken for estimate the effect of cleaning/ disinfecting in a critical ward (internal medicine). As reported by Siegel et al., we showed that the sites frequently touched by patients and healthcare workers (bed rails and bedsides tables) were the most commonly contaminated sites [12]. Boyces et al. have reported that environmental surfaces near patients such as bed rails, tray tables, telephones, bedside tables, patient chairs, and nurse call buttons are often heavily contaminated [13]. Furthermore, many bacteria as MRSA, A. baumannii, Pseudomonas spp., Salmonella spp. and VRE, have been associated with outbreaks and linked to contaminated mattresses, pillows and bed frames. In our ward, cleaning and disinfecting practices are conducted as a routine activity by environmental services workers, using a quaternary amine based product or bleach. However, we have likewise identified several bacteria of human and environmental origin, which is known to cause nosocomial infections. Coagulase Negative Staphylococci (CoNS) are isolated from 100% of sites studied, and are furthermore resistant to several antibiotics (penicillin and erythromycin 71%, methicillin and fusidic acid 57%). These opportunist bacteria are recognized as a cause of human infection including bacteremia and endocarditis particularly for immuno-compromised patients [13]. Also, when exposed to medical devices, CNS can form biofilms on polymer surfaces and devitalized tissue, making biofilm-associated CoNS more resistant to penetration by antimicrobial agents [14]. In our study, 0.16% of environmental surfaces was contaminated by MRSA (2/112), isolated from bedrails and rooms door knobs. This bacterium stills a major concern for hospital hygiene and represents one of the leading causes of hospital-acquired infection. We have also isolated 2 VRE from the bedrails (0.16%). Other authors have recovered this resistant pathogen at 16% in hospital room surfaces; even though, standard terminal cleaning protocols had been well followed [15]. Also, Enterobacteriaceae (10 K. pneumoniae 2 E. coli and 18 Enterobacter sp.) were present on 39.2% of analyzed surfaces. From these, 1 isolate of E. aerogenes are ESBL producing and 4 E. cloacae are resistant to Cephalosporin third generation. Worldwide, E. cloacae and E. aerogenes have been regularly involved in nosocomial infections outbreaks. They are considered as a part of the ESKAPE pathogens including: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species. These bacteria represent the vast majority of isolates whose resistance to antimicrobial agents represents a serious therapeutic dilemmas for physicians [16]. Pantoea spp. was also isolated (12 isolates). Actually, several studies have reported the association of this germ with nosocomial infections [17,18]. In the other hand, P. aeruginosa haven't been isolated but we identified 10 isolates of P. fluorescens resistant to ticarcillin and 4 isolates of S. malthophilia. These species have acquired increasing importance as nosocomial pathogens and have been involved in many nosocomial infections outbreaks in immunecompromised patients [19].
A multiple studies have reported that cleaning and disinfection protocols were frequently inadequate [8]. Despite this, environmental cleaning may be regarded as a fundamental principle of infection prevention in healthcare settings (1). More, all hospitals should provide a written protocol of cleaning and develop programs to optimize the thoroughness of high touch surface cleaning as part of terminal room cleaning at the time of discharge or transfer of patients [20]. The results of our study will be used as pedagogical tool for hospital staff to raise awareness about hospital cleaning process and to ensure that environmental cleaning procedures must be followed consistently.
Conclusion
We report in this study that hospital environment is contaminated by a variety of pathogenic and opportunist, resistant and sensitive bacteria isolated from many surfaces sites of patients rooms. Hospital must implement evidencebased infection prevention measures that will reduce the risk of transmission of pathogens via contaminated hospital surfaces and medical devices.
Acknowledgment
The authors thank all persons who have contributed in the realization of this work notably the hospital director and the head of service concerned by the study.
References
- Dancer S. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009; 73: 378-385.
- Palmore TN, Henderson DK. Controlling the Spread of Resistant Pathogens in the Intensive Care Unit. Chapter 89, in Mayers, D (ed) Antimicrobial Drug Resistance. Humana Press, Springer Publishing, New York, NY. 2015
- Otter J, Yezli S, Salkeld J, French G. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control 2013; 41: 6-11.
- Villegas MV, Hartstein AI. Acinetobacter outbreaks, 1977-2000. J Infect Control Hosp Epidemiol 2003; 24: 284-295.
- Huang SS, Datta R, Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. J Arch Intern Med 2006; 166: 1945-1951.
- Shaughnessy MK, Micielli RL, DePestel DD. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. J Infect Control Hosp Epidemiol 2011; 32: 201-206.
- Chemaly Roy F, Sarah S, Charles D, Shashank S, Ghantoji S, Maria R, Julie G, Julie S, Mark S. The role of the healthcare environment in the spread of multidrug-resistant organisms: update on current best practices for containment. J Ther Adv Infect Dis 2014; 2: 79-90.
- Han JH, Sullivan N, Leas BF, Pegues DA, Kaczmarek JL,Umscheid CA. Cleaning Hospital Room Surfaces to Prevent Health Care-Associated Infections. Ann Intern Med 2015; 163: 598-607.
- Dancer S. Controlling Hospital-Acquired Infection: Focus on the Role of the Environment and New Technologies for Decontamination. J Ther Adv Infect Dis 2014; 27: 665-690.
- Dancer S. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. J Lancet Infect Dis 2008; 8: 101-13.
- Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health Care Infection Control Practices Advisory C: Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am. J Infect Control 2007; 35: S65-S164.
- Boyce JM. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect 2007; 65: 50-54.
- Chu VH, Woods CW, Miro JM, Hoen B, Cabell CH, Pappas PA, Federspiel J, Athan E, Stryjewski ME, Nacinovich F, Marco F, Levine DP, Elliott TS, Fortes CQ, Tornos P, Gordon DL, Utili R, Delahaye F, Corey GR, Fowler VG. Emergence of coagulase-negative staphylococci as a cause of native valve endocarditis. J Clin Infect Dis 2008; 46: 232.
- Tristan A, Lina G, Etienne J, Vandenesch F. Biology and pathogenicity of staphylococci other than Staphylococcus aureus and Staphylococcus epidermidis, In: Fischetti, V.A., et al (Eds.),Gram-Positive Pathogens. E-Publishing ASM Press.,Washington, D.C. 2006; 572-86.
- Byers K, Durbin L, Simonton B,Anglim A, Adal K, Farr B. Disinfection of hospital rooms contaminated with vancomycin-resistant Enterococcus faecium. J Infect Control Hosp Epidemiol 1998; 19: 261-264.
- Rice LB. Progress and challenges in implementing the research on ESKAPE pathogens. J Infect Control Hosp Epidemiol 2010; 1: 7-10.
- Liberto MC, Matera G, Puccio R, Russo TL, Colosimo E, Foca E. Six cases of sepsis caused by Pantoea agglomerans in a teaching hospital. J New Microbiol 2009; 32: 119-123.
- Tiwari S, Beriha SS. Pantoea species causing early onset neonatal sepsis: A case report. J Medical Case Reports 2015.
- Samonis G, Karageorgopoulos DE, Maraki S, Levis P, Dimopoulou D, Spernovasilis NA. Stenotrophomonasmaltophilia Infections in a General Hospital: Patient Characteristics, Antimicrobial Susceptibility, and Treatment Outcome. PLoS ONE 2012; 7: e37375.
- Guh A, Carling P. Options for Evaluating Environmental Cleaning. Healthcare-associated Infections (HAIs). Centers for Disease Control and Prevention 2010.