- Biomedical Research (2006) Volume 17, Issue 3
Enhancement of ENT Routine Diagnosis and Staging of Head and Neck Tumors using 18FDG-PET
Ali Maeed Al-Shehri*Department of ENT, College of Medicine, King Khalid University, P.O. Box 641, Abha, Saudi Arabia
- Corresponding Author:
- Ali Maeed Al-Shehri
Department of ENT, College of Medicine
King Khalid University P.O. Box 641, Abha, Saudi Arabia
E-mail: namas1@hotmail.com
Accepted Date: August 31, 2006
Abstract
The main indications for Fluoro-deoxyglucose-position-emission tmomography (FDG-PET) in head and neck tumors (HNT) are the primary staging of high-risk patients e.g. Primary Tumur size 2 (3pT2) with an emphasis on the evaluation of lymph node involvement and presence or absence of organ metastases, an assessment of suspected recurrence, the evaluation of local or distant metastases and the search for primary tumors in CUP-syndrome. An ongoing prospective study was conducted covering 124 patients of both sexes between the ages of 34-72 years. Fifty-six patients were suspected of recurrence, 53 patients showed a staging due to large primary and patients with CUP-syndrome. Primary tumour localizations showed a majority of oropharynx (n=55) followed by larynx (n=20), hypopharynx (n=10), etc. Histologically most cancers were squamous cell carcinoma. In this study in 27% of patients with CUP syndrome, FDG-PET could identify the primary tumour whereas CI has failed. The data of this study as well as previous investigations indicate FDG-PET as a valuable diagnostic tool in the assessment and therapy management of head and neck tumors. FDG-PET exerts a reproducible high sensitivity and accuracy under clinical routine conditions within an appropriate selected patient cohort. Qualitative image assessment employing reference organs seems to be sufficient for reliable interpretation of FDG-positive lesions. Due to the high glucose utilization of HNT it is possible to delimit even small processes of >5-7 mm, although micrometastases <5-7 mm could not be detected.
Keywords
FDG-PET, head and neck tumors, CUP syndrome, carcinoma, micrometastases
Introduction
The application of computerized tomographic examination methods is a non-invasive method for surveying the whole body, and allows the exact localization of specific sites of the body. Oral or parenteral applied radioactive substances distribute into the organism corresponding to their physicochemical properties. In case of functional disturbances, metabolic disorders, defective parenchyma and other pathological aberrations deviation from normal concentration distribution can be seen. [1-4]
For clinical application of Positron Emission Tomography (PET) in most cases radioactive nuclides are produced in nuclear centers near to the applying institutions. Charges are a limiting factor for a PET center including a cyclotron, because they are much higher than for a PET hospital which receives their radionuclides from a nearby PET center or a clinical research center. [2-8]
Number, size, density and resolution of the detectors determine image reconstruction. Very often block detectors are bound into a ring system and switched together so to demonstrate the investigated structures in one or more planigraphic planes. Superior measurements show local spatial resolutions of <2 mm every second. Bismuth Gurmanate Oxide (BGO) crystals needed for the detection of photons are now of constant quality. For maximum demand BaF2 crystals are used. [9-10]
Reproducibility of the examination depends on technical standards as well as on specific factors of the examined patient so as physical status, actual metabolism including blood sugar profile and environmental influences. [8-11] For the labeling of biomolecules and pharmaceutics the isotopes 11C, 13N und 15O are applied, as they do not change their physicochemical characteristics in organisms. They can be manufactured with a low energetic particle accelerator, but because of their short radioactive half-life, production has to be done close to application. 18F is of clinical importance, too, as it can substitute hydrogen and hydroxyle groups, and because of its extended half-life it can be applied also in satellite centers. Application of 18FDG is a standard method for metabolic investigations. [2-14]
In oncology priority is given to early diagnosis. Limiting factors are the charges and the number of PET centers, but the applications for Fluoro-deoxyglucose-positionemission tomography (FDG-PET) imaging are rapidly growing and accepted in the field of oncology [15] For the diagnosis of skeletal metastases 18F-fluoride is used. 18F-FDG is a valuable supplemented tool for routine checkup in high-risk patients for detecting various malignancies. [16]
Until now PET studies described tumor perfusion, determination of synthesis ratio and aggressivity via investigation of the tumor metabolism, therapeutical planning, recidive diagnosis and documentation of postoperative scars. Determination of the locally changed perfusion and metabolism in tumor tissue is possible. The deconjugation of glucose uptake and reduced oxygen metabolism in anaerobe glycolysis can be proved. In-vivo investigations of the pharmaco-kinetics of cytotoxic substances may afford an insight into uptake, distribution, retention and toxicity of these substances.
Staging of head and neck tumors using 18FDG -PET The extension of malignant tumors depends on the localization, size, and histology of the primary cancer, which is conventionally diagnosed with methods such as physical examination, endoscopy and imaging techniques as ultrasound examination, computed tomography (CT) and magnetic-resonance tomography (MRT). Depending on the diagnostic procedure, the primary therapeutic approach, including surgery and radiation, should be a curative one even in the face of large tumors. This calls for a most specific and accurate diagnosis, leading to a complete local tumor resection, including lymph node metastases, which renders cure in a high percentage, whereas incomplete resection as well as unsuspected lymph node metastasis of other regions is associated with early tumor recurrence. However, the identification of the primary cancer in CUP-syndrome (cancer of unknown primary) [9, 10] or the early diagnosis of tumor recurrence have a better prognosis as well.
The analysis of cancer in the head and neck region identifies nearly 85-90% of newly diagnosed malignant tumors of this region as squamous cell carcinoma. Others are rare cancers as adenocarcinoma, lymphoma, sarcoma, and lympho-epithelial tumors.
Conventional examination methods often lead to a diagnosis of inadequate specificity. Even functional imaging methods, including tumor seeking substances as, for example, specific monoclonal antibodies were not able to improve the diagnostic accuracy. [17] 18Fluoro- Deoxyglucose shows comparably high concentrations in brain, heart (nonfasting state), highly malignant tumors and inflammatory or regenerative processes. The feasibility of FDG-PET in head and neck tumors was demonstrated under study conditions with results of high sensitivity, specificity and accuracy. [18-21]
This investigation aimed to define tumor-specific FDGuptake for qualitative image evaluation, assessment of the diagnostic accuracy as compared to conventional imaging, and specification of selection criteria for patients eligible for a FDG-PET study.
Material and Methods
An on-going prospective study was conducted covering 124 patients of both sexes between the ages of 34-72 years.
After routine examination all the patients scheduled for surgery due to a clinically suspected or biopsy proven malignancy were eligible for an FDG-PET study. The patients had given informed consent prior to study enrollment and were aware of the study character of the investigation. All unsuspected findings were tried to be confirmed either by other imaging modalities, biopsy or surgery to identify local or regional tumor spread, and/or clinical course as for e.g. in the case of distant metastases. The patients were followed up for at least 6 months and routinely checked on a 3-monthly bases. The final clinical diagnosis was used as gold standard.
Whole body examinations from the base of the skull to the pelvis were performed with a dedicated PET-scanner (ECAT EXACT 921/47; Siemens-CTI, Knoxville, TN, USA) with a field of view (FOV) of 16.2 cm producing 47 simultaneous transaxial slices at each longitudinal bed position with a slice thickness of 3.2 mm in the transaxial projection.
CT-scans were routinely performed on a spiral-CT (Philips, Germany) with and without contrast enhancing agent. MRI was performed on a 1 or 1.5 Tesla Gyroscan (Philips, Germany) with and without the contrast enhancing agent Gadolinium.
The patients fasted for at least 12-18 hrs prior to injection of 185-260 MBq and were scanned 45-60 min. after injection (10 min. per FOV). 18FDG was commercially obtained by the Research Center of Karlsruhe (Germany) or Juelich (Germany). In order to allow measured attenuation correction, a whole body transmission scan was routinely performed prior to FDG-injection (7-10 min. per FOV), using a rotating 68 Ge/ 68 Ga-source. Emission data were reconstructed by filtered back projection with a Hanning filter, a cutoff frequency of 0.4/cycle without scatter- but decay-correction. Scans were corrected for attenuation based on the measured transmission data. Results were displayed on computer screen as three orthogonal images allowing interactive choice of slice localization by the investigator. Image evaluation was primarily performed on screen. Transaxial, coronal and sagittal views were documented black and white paper prints. In order to facilitate visual scoring, pathological findings were printed also in color code (rainbow) with the cerebellum set to maximum.
Images were read by two experienced nuclear medicine (PET) and radiology (CT, MRI) physicians, which were not fully blinded to available data (diagnostics, results of prior studies etc.), but had no knowledge of the results of the other imaging studies being employed.
Results
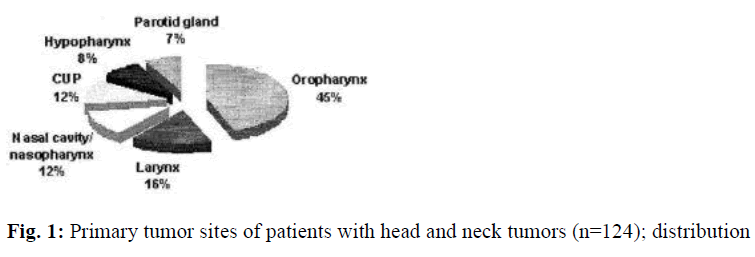
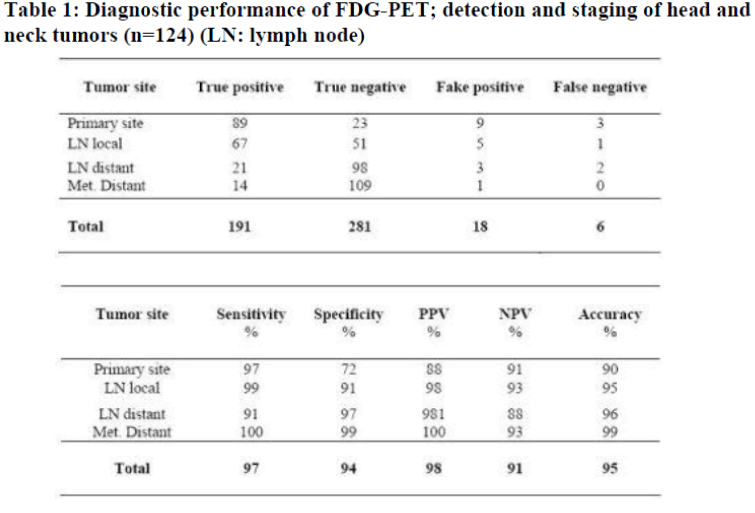
In the ongoing prospective study, 85 male and 39 female patients between 34 and 72 years of age underwent FDGPET. 56 patients were suspected recurrence, 53 patients showed a staging due to large primary, and 15 patients with CUP-syndrome. Primary tumor localizations were oropharynx (n=55), larynx (n=20), hypopharynx (n=10), parotid gland (9), nasal cavity (n=8), nasopharynx (n=7), and CUP (n=15). Histologically most cancers were squamous cell carcinoma (n=93, 75%) (Figs. 1, 2). FDG-PET was able to correctly identify 89 of 92 malignant lesions, with 3 lesions being false negative (Table 1). False negative findings were due to chemotherapy (n=2) with low uptake in tumor tissue and a tumor growing along the mucosa of the oral cavity (n=1; tumor thickness below 2 mm). The qualitative assessment of FDG-uptake did not reveal an evident difference between primary versus recurrent tumor tissue. In nine cases, scanning shortly after surgery (<10 days) showed focal areas with high FDG-accumulation in the operated area, which were misinterpreted as tumors of the parotid gland presented with high FDG-uptake (n=5). The histological investigation identified them as pleomorphic adenoma and thus false positive. In 23 of 26 cases, FDG-PET correctly predicted the absence of a malignant process. Overall, sensitivity and accuracy were calculated to be 97% and 90% , respectively (Table 1).
Many patients with pT2 tumors (36%) or recurrence (49 %) presented with tumor spread to cervical lymph nodes. In 67 of 72 cases FDG-PET correctly identified cervical lymph node involvement. In 1 case, cervical lymph node involvement was not identified, and in 5 cases the result was false positive (Table 1). These data represent a sensitivity and accuracy of 99% and 95% , respectively. In addition, PET detected 21 of 24 distant lymph node metastases in lung, liver, or bone, which were correctly identified in 14 of 15 cases and excluded in 109 cases.
In the group of patients with CUP-syndrome (n=15), FDG-PET was able to visualize 4 (27%) prior unidentified primary tumors and correctly predicted further local (lymph node) metastases in 7 (47%), or distant metastases in 4 (24%) patients. Three (20%) showed no evidence of disease and are currently followed up. Thus, the detection sensitivity of an unknown primary cancer by FDG-PET was 27% , exceeding that of any other imaging modality (sensitivity 0%).
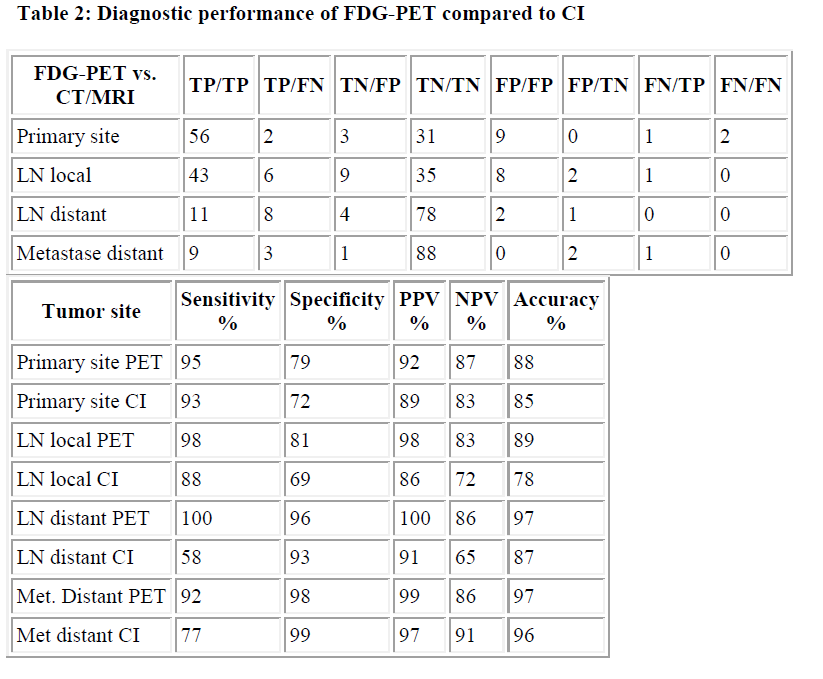
Direct comparison of the diagnostic performance and safety of FDG-PET versus conventional imaging (CI=CT/MRI) in 104 patients are summarized in Table 2. The data demonstrate a high agreement in the detection of the various lesions: 119 of 141 lesions were detected by both techniques (Table 2). 19 of 141 lesions (14%) were only detected by FDG-PET, whereas 3 of 141 (2%) were identified only by CI. In addition, FDG-PET correctly excluded tumor involvement in 17 lesions, which were false positive in 5 cases, but true negative in CI. Only in 2 cases both methods failed to detect proven tumors, in 19 lesions both methods proved to be falsepositive (Table 2): 9 of 19 lesions were localized in the primary tumor site, with 5 being histologically pleomorphic adenoma, while in 4 lesions no tumor recurrence could be established; 10 of 19 lesions were assumed to be lymph node metastases, but could not be verified histologically. The use of FDG-PET significantly improved the correct staging of local and distant lymph nodes by roughly 10% as compared to CI (Table 2). Its impact on the primary tumor site was marginal with the exception of tumor recurrence and CUP-syndrome (data not shown). Nevertheless, while FDG-PET is routinely used for whole body or body trunk scanning as compared to defined regionscanning in CT or MRI, more distant metastases were primarily identified in our patient group underscoring the screening potential of this method in selected high-risk patients.
Discussion
FDG-PET seems to be very helpful in specific situations where computer tomography (CT) has known limitations, such as differentiation of benign from malignant indeterminate lesions on CT, differentiation of post-treatment changes versus recurrent tumor, differentiation of benign from malignant lymph nodes, and monitoring therapy. The biggest use of FDG-PET presently is in staging of various body tumors. [15] Initial studies of extensive HNT showed high FDG-accumulation in histologically verified tumor tissue [3,4,22-24] and high contrast as compared to normal tissue or the normal contralateral site.
They also had demonstrated high FDG-uptake in recurrent tumors as well as in lymph node and organ metastases. Non-malignant sites usually showed moderate FDGuptake only and diminished contrast to surrounding tissues. In this study the application of FDG-PET in the staging of head and neck tumors (HNT) in selected patients under routine conditions was investigated. Patients with pT>=2 tumors in the head/neck area show an avide FDGabsorption in vital tumor tissue in contrast to necrotic areas or benign anomalies. Even in large tumors a relatively homogeneous accumulation can be observed in the entire tumor mass. However, pleomorphic adenomas or lymphadenopathy caused by toxoplasmosis showed significant FDG-accumulations, too, and therefore may be misinterpreted as a malignant process. [6,7] The qualitative scoring of FDG-accumulation in histologically identified cancer compared to reference organs (cerebellum, mediastinum, liver), suggested cerebellum-like glucose utilization to be an indicator for a malignant process, [25] whereas inflammatory processes usually showed FDGutilization comparable to liver or mediastinum. Metastases, which were located in these regions, too, presented with high contrast allowing tumor detection with high sensitivity and accuracy. The use of reference organs for interpretation of focal FDG-uptake seems to be an easy and reliable method, while reducing misinterpretation by threshold manipulation. Most malignant lesions present with FDG-uptake comparable to cerebellum. As in this study patients with acute inflammatory processes were excluded, FDG-PET allowed a differentiation of tumor manifestation versus chronic inflammations with a high accuracy.
Using the above mentioned scoring system in high-risk patients, the high sensitivity and accuracy of FDG-PET in the functional assessment of tumor-masses, morphological changes and the presence of lymph node and/or organ metastases was proved. The data of this study clearly indicate, that FDG-PET, as compared to CI, has its main advantage in the evaluation of lymph node involvement, in the differentiation of postsurgically changed or distorted anatomy, and in the whole body scanning for the detection of distant metastases as well as in CUPsyndrome. According to previous studies the investigation showed, that under routine clinical conditions FDG-PET can be employed with a high sensitivity and accuracy.
The data showed the benefit of FDG-PET especially in the correct staging of lymph node involvement of CI in the previous findings. [5,11,12,23,24,26] A major limitation of CI in the assessment of normal sized or slightly enlarged lymph nodes is the lack of specificity. FDG-PET primarily depends on the relative metabolic rate, [1,12,23,24] which seems to be correlated with the proliferation index [27-29] Application of FDG-PET in this patient cohort correctly predicted in 14/69 (22%) or excluded in 15 cases lymph node involvement, contrarily to CI, thus significantly improving the overall diagnostic accuracy, and exerting direct effects on the therapeutic management.
Due to the high glucose utilization of these tumors it is possible to even safely delimit small processes such as lymph node metastase >5-7 mm or distant metastases (lung, liver, bones). [30] Micrometastases (<5-7 mm) mostly could not be detected. Studies of the diagnostic usefulness of FDG-PET following neck dissection showed a 96% sensitivity and 92% specifity in comparison to CT/MRI [13,16,31-34]. In another study direct comparison of the diagnostic precision of FDG-PET, CT, and clinical examinations following neck dissection resulted in comparable values for FDG-PET (82%) and CT (84%), but showed a considerable improvement as against clinical examinations (71%). A study recently published of resected tumors involving 1284 lymph nodes and 117 lymph node metastases showed a sensitivity and specificity of 90% and 94% respectively for FDG-PET. For CT and MRT, sensitivity was 82% and 80% respectively, and specificity was 85% and 79% respectively, which corresponds to the result of previous studies, [3,4,22,35] where FDG-PET and CT led to identical findings in 122 out of 141 histologically verified lymph node groups (87%). In 19 lymph node groups (13%), which showed no anomaly according to CT criteria, metastases could be identified using FDG-PET, while 17 lymph node groups (12%), despite positive CT findings, were classified as being tumor-free since there was no FDGaccumulation. Hence, in retrospect, FDG-PET in approx. 25% of all patients examined had an influence on the final evaluation of tumor spreading with an increase in diagnostic precision by more than 10% . Since FDG-PET examination is a total body examination, involvement of distal (mediastinal) lymph node groups could additionally be detected in 14% of patients waiting for surgery, and distant metastases (lung, liver, bones) were already found in 9% .
Overall, experience shows that FDG-Pet should be used before surgery, due to its high precision, for staging cervical lymph nodes and excluding distant metastases. FDG-PET allows an improved judgment of suspect CT findings with regard to their dignity, as a certain percentage of patients can be identified, who have lymph node metastases, which are tumor-free according to CT criteria or for whom curative surgery is no longer indicated due to the spreading of the tumor.
After surgery the situation is similar to identify tumor rests or a tumor recurrence. In case of postsurgical changes, morphological imaging cannot differentiate between anatomic deviation and recurrence, unless tumor growth definitely has been established. Since scar tissue does not show an increased glucose utilization, FDGaccumulation is most probably a consequence of vital tumor tissue, whereas a tumor recurrence can largely be excluded if there is no FDG-accumulation. [11,12,36,37] When directly comparing FDG-PET with clinical examinations or morphologic imaging, FDG-PET showed a considerably higher diagnostic precision. [1-3,14,22,38] In this study recurrence showed an intense FDG-uptake compared to primary tumors or lymph node metastases. These findings can be explained by the fact, that FDGtrapping correlates well with the proliferation index at least in HNT. [27,28,36] FDG-PET therefore allows an early detection of recurrence. [27,28,36,37,39] The additional functional information of “malignancy typical glucose utilization” provides much more safety with regard to the interpretation of the local finding, in particular for the ENT surgeon. Moreover, it could be observed that lymph node and/or distant metastases can be additionally detected.
Concerning therapy monitoring the data underline the possibility of an improved staging and therapy management when integrating FDG-PET in order to early subject the patients to an optimum therapy. [6,7,35,40] As some studies show, the amount of FDG uptake seems to correlate strongly with response to therapy, so that such findings may prevent from unnecessary therapeutical measures, which may lead to aggravation of the disease. [41] In a study within a standardized protocol, the authors found that FDG-PET recognized treatment response to radiation therapy in oral squamous cell carcinoma with a reasonable specificity and thus provides a basis for further therapeutic decisions. [42] In addition it could be found that the high accuracy of FDG-PET makes it a cost effective radiologic procedure in the workup of all suspected and/or recurrent cancer patients. [40,43]
The neck lymph nodes are a common site of metastases from carcinoma of unknown primary (CUP). Detection of the primary tumor in patients with cervical lymph node metastases is of considerable prognostic importance, as the primary tumor can often be removed with curative effect. Experience published shows that FDG-PET is also superior to morphologic examinations with regard to the technique of total body examination and, in 20-50% of the cases, capable of identifying a primary tumor which could not be found in previous examinations. [2,4,9,10,18,23,24,44] In this study, in 27% of the patients with CUP-syndrome FDG-PET could identify the primary tumor, whereas CI had failed. In previous studies with 25 patients, the primary tumor could be detected in 6 cases (24%), using FDG-PET. Here, too, it is remarkable that distant metastases were already detectable in 8 cases (32%), which were first identified within the scope of a total body examination using FDG-PET. [6,7,23,24] These findings support the postulation to establish this method in CUP-syndrome, when conventional diagnostic workup as well as CI fails to identify the primary tumor.
Conclusion
From this study, it can be inferred that FDG-PET is a valuable diagnostic tool in the assessment of head and neck tumors. FDG-PET exerts a reproducible high sensitivity and accuracy under clinical routine conditions within an appropriate selected patient cohort. Qualitative image assessment reference organs seems to be sufficient for reliable interpretation of FDG-positive lesions. Due to the high glucose utilization of HNT it is possible to delimit even small processes of >5-7 mm, although micrometastases <5-7 mm could not be detected. The main indications for FDG-PET in HNT are the primary staging of high-risk patients (3pT2) with an emphasis on the evaluation of lymph node involvement and presence of absence of organ metastases, an assessment of suspected recurrence, the evaluation of local or distant metastases and the search for primary tumors in CUP-syndrome.
Acknowledgement
Staging of Head and Neck Tumors using 18FDG-PET The author would like to acknowledge Prof. Mohd. Yunus Khan of the Department of Family and Community Medicine for reviewing the manuscript and Mr. Allan Agaton for typing out the article.
References
- Anzai Y, Carroll WR, Quint DJ, Bradford CR, Minoshima S, Wolf GT, Wahl R. Recurrence of head and neck cancer after surgery or irradiation: prospective comparison of 2-deoxy-2-[F-18]fluoro-D-glucose PET and MR imaging diagnoses. Radiology 1996; 200: 135- 141.
- Austin JR, Wong FC, Kim EE. Positron emission tomography in the detection of residual laryngeal carcinoma. Otolaryngol Head Neck Surgery 1995; 113: 404-407.
- Bailet JW, Abemayor E, Jabour BA, Hawkins RA, Ho C, Ward PH. Positron-emission tomography: a new, precise imaging modality for detection of primary head and neck tumors and assessment of cervical adenopathy. Laryngoscope 1992; 102: 281-288.
- Bailet JW, Sercarz JA, Abemayor E, Anzai Y, Lufkin RB, Hoh CK. The use of positron emission tomography for early detection of recurrent head and nack squamous cell carcinoma in postradiotherapy patients. Laryngoscope 1995; 105: 135-139.
- Benchaou M, Lehmann W, Slosman DO, Becker M, Lemoine R, Rufenacht D, Donath A. The role of FDG PET in the preoperative assessment of N staging in head and neck cancer. Acta Ololaryngol Stockh 1996; 116: 332-335.
- Bender H, Straehler-Pohl HJ, Linke D, et al. Klinische Bedeutung von 18-FDG-PET in der Diagnostik von Kopf-Hals-Tumoren. Nuklearmedizin, 1998, 37: 30-33.
- Bender H, Straehler-Pohl HJ, Schomburg A, et al.: Value of F-18-DG-PET in the assessment of head and neck tumors, J Nucl Med, 1997, 38: 153-156.
- Biersack HJ, Bender H, Ruhlmann J, Schomburg A, Grünwald F: FDG PET in clinical oncology: Review and evaluation of results of a private clinical PET center. In: Freeman LM (eds): Nuclear Medicine Annual 1997. Lippincott Raven Philadelphia, 1997 pp. 1-29.
- Bohuslaviszi KH, Klutmann S, Sonnemann U, Thoms J, Kroeger S, Werner JA, Mester J, Clausen M: F-18- FDG-PET for detection of occult primary tumor in patients with lymphatic metastases of the neck region. Laryngo-Rhino-Otologie 1999; 8: 445-449.
- Bohuslavizki KH, Klutmann S, Krueger S, SOnnemann U, Buchert R, Werner JA, Mester J, Clausen M. FDG PET detection of unknown primary tumors. J Nucl Med, 2000; 41: 816-822.
- Braams JW, Pruim J, Freling NJ, Nikkels PG, Roodenburg JL, Boering G, Valburg W, Vermay A. Detection of lymph node metastases of squamous cell cancer of the head and neck with FDG PET and MRI. J Nucl Med 1995; 36: 211-216.
- Braams JW, Pruim J, Kole AC, Nikkels PG, Vaalburg W, Vermey A, Roodenburg JL. Detection of unknown primary head and neck tumors by positron emission tomography. Int J Oral Maxillofac Surg 1997; 26: 112- 115.
- Chisin R, Macapinlae HA: The indication of FDG-PET in neck oncology. Radiol Clin North Am 2000; 38: 999- 1012.
- Daldrup-Link HE, Franzius C, Lin TM, Laukamp D, Sciuk J, Juergens H, Schober O, Rummeny EJ. Wholebody MR imaging for detection of bone metastases in childen and young adults: comparison with skeletal scintigraphy and FDG-PET. AJR 2001; 177: 229-236.
- Delbeke D, Martin WH: Positron emission tomography imaging in oncology. Radiologic Clinics of North America 2001; 39: 883-917.
- Kao CH, Kwan AS, Kwan JK, Chow MJ. The role of 18F-fluorodeoxyglucose positron emission tomography in cancer screening - a preliminary report. Oncology Reports 2001; 8: 1145-1148.
- Watkinson JC: Nuclear medicine in otolaryngoloy. Clin Otolaryngol 1990; 15: 457-469.
- Jungehuelsing M, Scheidhauer K, Damm M, Pietrzyk U, Eckel H, Schicha H, Stennert E. 2(f)-fluoro-2-deoxy-Dglucose positron emission tomography is a sensitive tool for the detection of occult primary cancer (carcinoma of unknown primary syndrome) with head and neck lymph node manifestation. Otolaryn - Head Neck Surg 2000; 123: 294-301.
- Nowak B, DiMartino E, Jaenicke S, Cremerius U, Adam G, Zimny M, Reinartz P, Buell U. Diagnostic evaluation of malignant head and neck cancer by F-18-FDG Pet compared to CT7MRI. Nuklearmedizin 1999; 38: 312- 318.
- Stokkel MP, ten Broek FW, Hordijk GJ, Kolle R, van Rijk PP: Preoperative evaluation of patients with primary head and neck cancer using dual-head 18fluorodeoxyglucose positron emission tomography. Ann Surg 2000; 231: 229-234.
- Stokkel MP, Terhaard CH, Hordijk GJ, van Rijk PP. The detection of unknown primary tumors in patients with cervical metastases by dual-head positron emission tomography. Oral Oncol 1999; 35:: 390-394.
- McGuirt WF, Williams DW 3rd, Keyes JW jr, et al.: A comparative diagnostic study of head and neck nodal metastases using positron emission tomography. Laryngoscope 1995; 105: 373-375.
- Wong WL, Chevretton EB, McGurk M, et al. A prospective study of PET-FDG imaging for the assessment of head and neck squamous cell carcinoma. Clin Otolaryngol 1997; 22: 209-214.
- Wong WL, Hussain K, Chevretton E, Hawkes DJ, Baddeley H, Maisey M, McGurk M: Validation and clinical application of computer combined computed tomography and positron emission tomography with 2- head and neck images. Am J Surg 1996; 172: 628-632.
- Zeitouni A, Yamamoto YL, Black M, Gjedde A. Functional imaging of head and neck tumors using positron emission tomography. J Otolaryngol 1994; 23: 77-80.
- Jabour BA, Choi Y, Hoh CK, Rege SD, Soong JC, Lufkin RB, Hanafee WN, Maddahi J, Chaiken L, Bailet J, et al. Extracranial head and neck: PET imaging with 2-[F18]-fluoro-2-deoxy-D-glucose and MR imaging correlation. Radiology 1993; 186: 27-35.
- Minn H, Clavo AC, Grenman R, Wahl RL. In vitro comparison of cell proliferation kinetics and uptake of tritiated fluorodeoxyglucose and L-methionine in squamous cell carcinoma of the head and neck. J Nucl Med 1995; 36: 252-258.
- Minn H, Joensuu H, Ahonen A, Klemi P. Fluorodeoxyglucose imaging: a method to assess the proliferative activity of human cancer in vivo. Comparison with DNA flow cytometry in head and neck tumors. Cancer 1988; 61: 1776-1781.
- Reisser C, Haberkorn U, Strauss LG. The relevance of positron emission tomography for the diagnosis and treatment of head and neck tumors. J Otolaryngol 1993; 22: 231-238.
- Gupta NC, Graeber GM, Bishop HA. Comparative efficacy of positron emission tomography with fluorodeoxyglucose in evaluation of small (>1 cm), intermediate (1 to 3 cm), and large (>3 cm) lymph node lesions. Chest 2000; 117: 773-778.
- Kao CH, Hsieh JF, Tsai SC, Ho YJ, Yen RF, ChangLai SP, Chieng PU. Comparison of 18-fluoro-2-deoxyglucose positron emission tomography and computed tomography in detection of cervical lymph node metastases of nasopharyngeal carcinoma. Ann Otol Rhinol Laryngol 2000; 109: 1130-1134.
- Kao CH, Tsai SC, Wang JJ, Ho YJ, Yen RF, Ho ST. Comparin 18-fluoro-2-deoxyglucose positron emission tomography with a combination of technetium 99m tetrofosmin single photon emission computed tomography and computed tomography to detect recurrent or persistent nasopharyngeal carcinomas after radiotherapy. Cancer 2001; 92:: 434-439.
- Pichler R, Maschek W, Hatzl-Griesenhofer M, Huber H, Wimmer G, Wahl G, Fridrik M. Klinische Wertigkeit der Befunde von FDG-PET mittels Koinzidenz- Gammakamera beim Staging and Restaging maligner Lymphome - ein Vergleich zu konventioneller Diagnostik. (Clinical value of FDG PET using coincident gamma cameras in staging and restaging of malignant lymphoma - compared with conventional diagnostic methods.) Nuklearmed 2000; 39: 166-173.
- Sutinen E, Jyrkkioe S, Varpula M, Lindholm P, Groenroos T, Lehikoinen P, Teraes M, Minn H. Nodal staging of lymphoma with whole-body PET: comparison of. J Nucl Med 2000; 41: 1980-1988.
- Straehler-Pohl HJ, Bender H, Linke D, et al. Value of F- 18-DG-PET in the assessment of head and neck tumors: Clinical experience in 152 patients. J Cancer Res Clin Oncol 1998; 124: R10.
- Haberkorn U, Strauss LG, Reisser C, Haag D, Dimitrakopoulou A, Ziegler S, Oberdorfer F, Rudat V, van Kaick G. Glucose uptake, perfusion, and cellproliferation in head and neck tumors: relation of positron emission tomography to flow cytometry. J Nucl Med 1991; 32: 1548-1555.
- Rege S, Maass A, Chaiken L, Hoh CK, Choi Y, Lufkin R, Anzai Y, Juillard G, Maddahi J, Phelps ME. Use of positron emission tomography with fluorodeoxyglucose in patients with extracranial head and neck cancers. Cancer 1994; 73: 3047-3058.
- Rigo P, Paulus P, Kaschten BJ, Hustinx R, Bury T, Jerusalem G, Benoit T, Foidart Willems J. Oncological application of positron emission tomogrpahy with fluorine 18 fluorodeoxyglucose. Eu J Nucl Med 1996; 23: 1641-1674.
- Li P, Zhuang H, Mozley PD, Denittis A, Yeh D, Machtay M, Smith R, Alavia A. Evaluation of recurrent squamous cell carcinoma of the head and neck with FDG positron emission tomography. Clin Nucl Med 2001; 26: 131-135.
- Tucker R, Coel M, Ko J, Morris P, Druger G, McGuigan P. Impact of fluorine-18 fluorodeoxyglucose positron emission tomography on patient management: first year’s experience in a clinical center. J Clin Oncol 2001; 19: 2504-2508.
- Schechter NR, Gillenwater AM, Byers RM, Garden AS, Morrison WH, Nguyen LN, Podoloff DA, Ang KK. Can positron emission tomography improve the quality of care for head-and-neck cancer patients? Int J Rad Oncol Biol Phys 2001; 51: 4-9.
- Kunkel M, Groetz KA, Foerster GJ, Wahlmann U, Benz P, Kutzner J, Rippins G, Wagner W. Therapiemonitoring mittels 2-(18F)-FDG-Positronenemissionstomographie nach neoadjuvanter Strahlenbehandlung des Mundhöhlenkarzinoms. (Therapy monitoring with 2-(18F)- FDG positron emission tomography after neoadjuvant radiation treatment of mouth carcinoma.) Strahlentherapie und Onkologie 2001; 177: 145-152.
- Lonneux M, Lawson G, Ide C, Bausart R, Remacle M, Pauwels S. Positron emission tomography with fluorodeoxyglucose for suspected head and neck tumor recurrence in the symptomatic patient. Laryngoscope 2000; 110: 1493-1497.
- Valk PE, Ponds TR, Tesar RD, Hopkins DM, Haseman MK. Cost effectiveness of PET imaging in clinical oncology. Nucl Med Biol 1996; 23: 737-743.