Research Article - Biomedical Research (2017) Volume 28, Issue 2
Endothelin-1 Lys198Asn and rs10478694 polymorphism in ischemic stroke
Orhan Aslan1, Mehtap Gurger2, Metin Atescelik2, Murat Kara3, Askin Sen4, Omer Dogan Alatas5,Caner Fevzi Demir6, Mustafa Yilmaz2*, Kursat Kargun71Department of Emergency Medicine, Gazi Yasargil Education and Research Hospital, Diyarbakir, Turkey
2Department of Emergency Medicine, Firat University School of Medicine, Elazig, Turkey
3Department of Medical Genetics, Sitki Kocman University Faculty of Medicine, Mugla, Turkey
4Department of Medical Genetics, Firat University Hospital Faculty of Medicine, Elazig, Turkey
5Department of Emergency Medicine Sitki Kocman University Faculty of Medicine, Mugla, Turkey
6Department of Neurology Firat University School of Medicine, Elazig, Turkey
7Elazığ High School of Health Sciences, Fırat University, Elazig, Turkey
Accepted on July 6, 2016
Abstract
Objective: In this study, we aimed to investigate the relationship between ischemic cerebrovascular diseases and Lys198Asn and rs10478694 polymorphism of Endothelin-1 gene, a strong vasoconstrictor.
Materials and Methods: Blood samples were isolated from 100 patients diagnosed with ischemic cerebrovascular diseases and Lys198Asn and rs10478694 polymorphism of Endothelin-1 gene from obtained DNAs by Real-Time Polymerase Chain Reactions Method (RT-PCR).
Results: Forty-one patients in the patient group and 38 patients in the control group were found to be heterozygous for Lys198Asn. Thirty-nine patients in the patient group and 39 patients in the control group were found to be heterozygous for rs10478694. Lys198Asn was found to be homozygous mutant in 8 patients in the patient group and 9 patients in the control group. Rs10478694 was found to be homozygous mutant in 9 patients in the patient group and 7 patients in the control group. There was no significant difference in age and gender between patients with and without identified polymorphisms (p>0.05). In addition, smoking and alcohol use, presence of hypertension, diabetes mellitus, coronary artery disease and chronic obstructive pulmonary disease was found not to be correlated with positivity of gene polymorphism.
Conclusion: Lys198Asn and rs10478694 polymorphism of Endothelin-1 gene was found not to be correlated with ischemic cerebrovascular diseases.
Keywords
Ischemic cerebrovascular disease, Endothelin-1, Lys198Asn, rs10478694.
Introduction
Cerebrovascular Diseases (CeVD) constitute about 10% of all emergency room admissions and vast majority of emergency room admissions with a neurological disease [1]. Age, gender, race, arterial hypertension, diabetes, Cardiovascular Diseases (CVD), hyperlipidaemia, smoking, alcohol, obesity, genetic predisposition, carotid or vertebral artery stenosis are important risk factors for ischemic cerebrovascular diseases [2]. The studies on risk factors, chemicals and genetic effects that have effect on development of CeVD are underway. Endothelin-1 is one of these substances.
Endothelins (ET), found in very small concentrations in the circulation, are 21-amino acid most potent vasoconstrictor polypeptides that are produced primarily in endothelium of vascular smooth muscle and that exert paracrine and autocrine effects [3]. They show a heterogeneous distribution in the central nervous system (CNS). While Endothelin-1 (ET-1) and 3 are found in neuronal tissue, ET-1 is more in the brain stem and endothelin-3 is more in the pituitary gland. Although the role of ETs is not known with certainty in brain, these are contemplated to be partially involved in the regulation of local cerebral blood flow and be neuropeptides [4]. Endothelin 1 levels in the blood and cerebrospinal fluid have been reported to be elevated in cerebrovascular diseases [5]. Genetic factors playing an important role in the development of CeVD lead to an increase in genetic studies that are aimed to detect susceptibility to this disease before the onset of disease [6,7]. Among the genetic studies, potential endothelial gene polymorphisms are also included. Many phenotypes were studied to examine the relationship between polymorphisms in ET Type A (ETa) (-231G>A, +122C>T) and ET Type B (ETb) receptor (G57S and L277L) in ET-1 gene (EDN1) (K198N) [8].
Two nucleotide polymorphisms in Endothelin-1 gene (EDN1) have been reported to be associated with blood pressure. The first of these is Lys198Asn polymorphism, which is known to play a role in development of hypertension and affect vascular reactivity [9-12]. The other one is rs10478694 polymorphism, which increases levels of ET-1, causes a significant imbalance in vascular tone and further triggers serious endothelial dysfunction [13-15].
We aimed to investigate phenotypic implications of Lys198Asn and rs10478694, which are found on endothelin-1 gene, in patients diagnosed with ischemic CeVD in the emergency department.
Materials and Methods
In our study, 100 patients with ischemic CeVD diagnosis and 100 control subjects without a known history of CeVD, acute renal failure and acute coronary syndrome were included. Accompanying haemorrhagic cerebrovascular disease, traumatic brain injury, acute renal failure or acute coronary syndromes were considered as exclusion criteria. In addition, those under 18 years of age and those who did not consent were excluded. The demographic data of patients were recorded into the standard data form. The local ethics committee approval was obtained for this study.
Two cc of venous blood samples were obtained from both study groups into Ethylenediaminetetraacetic acid (EDTA) tubes. Bloods were kept at -80ºC for approximately 7 days until DNA purification and tubes were studied after homogenization.
DNA analysis
Based on the standard DNA extraction protocols, obtained blood samples were isolated using Invitrogen brand DNA isolation kit. Rs10478694 and Lys198Asn polymorphism in Endothelin 1 gene were studied with Applied Bio systems Step One Plus device using Real-Time Polymerase Chain Reactions (RT-PCR).
Statistical analysis
Statistical Package for the Social Sciences (SPSS 21, Chicago, IL, USA) program was used for analysis of the data. Compliance of genetic distribution with Hardy-Weinberg equilibrium was analysed by X2 goodness-of-fit test. Differences of genotypic and allele distributions between patients and controls were evaluated with chi-square test. Results were evaluated with 95% confidence interval and significance was at p <0.05 level.
Results
Fifty-four (54%) patients in the study group and 67 (67%) patients in the control group were male. The mean age was 71.7 ± 10.6/year (range, 49-93 years) in patient group and 65.8 ± 7.3/year (range, 47-85 years) in control group. No significant difference was found between groups in terms of age and sex distribution (p>0.05). Habit profile of the patient group and the control group were similar. Seventy-six per cent of CeVD group and 79% of control group did not use alcohol or smoke. Given the background of the patient and control groups, hypertension (70%) was observed most often, followed by coronary artery disease (34%), atrial fibrillation (32%) and diabetes mellitus (28%). Eighteen patients from the patient group had CeVD before. Physical examination findings of the patients are shown in Table 1.
| Physical examination findings of the patients | |
|---|---|
| Mean GCS | 13.82 ± 1.87 (minimum 3-maximum 15) |
| Mean systolic blood pressure | 138.75±30.43 mmHg |
| Mean diastolic blood pressure | 78.98±15.87 mmHg |
| Hemiparesis (n) | 78 |
| Dysphasia (n) | 52 |
| Aphasia (n) | 25 |
| Facial paralysis (n) | 17 |
Table 1. Physical examination findings of the patients.
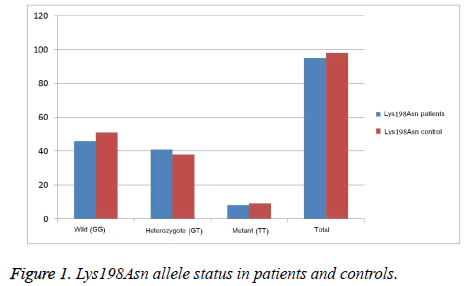
There was no significant difference between control and patient group in terms of genotype and allele distribution of Endothelin 1 as shown in Table 2. Regarding Lys198asn allele comparison between patient and control group; while 46 (48.42%) of the patients were wild homozygous (GG), 41 (43.16%) were heterozygous (GT) and 8 (8.42%) were homozygous mutant (TT) in the patient group, 51 (52.04%) were wild homozygous (GG), 38 (38.78%) were heterozygous (GT) and 9 (9.18%) were homozygous (TT) mutants in the control group. There were no significant differences between the groups (p=0.825) illustrated in Figure 1.
| Genotype Allele | Patient(n=95) | Control(n=98) | |||
|---|---|---|---|---|---|
| X2 | P | ||||
| Lys198Asn | GG | 46 (0.484) | 51 (0.520) | ||
| GT | 41 (0.432) | 38 (0.388) | 0.383 | 0.825 | |
| TT | 8 (0.084) | 9 (0.092) | |||
| G | 133 (0.700) | 140 (0.714) | 0.095 | 0.757 | |
| T | 57 (0.300) | 56 (0.286) | |||
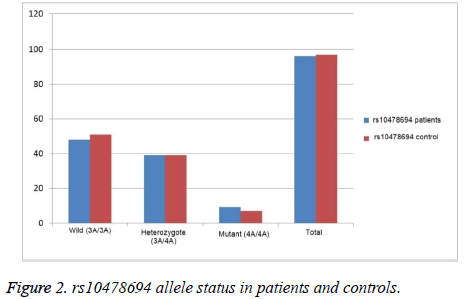
| Genotype Allele | Patient(n=96) | Control(n=97) | X2 | P | |
| rs10478694 | 3A/3A | 48 (0.500) | 51 (0.526) | 0.335 | 0.845 |
| 3A/4A | 39 (0.406) | 39 (0.402) | |||
| 4A/4A | 9 (0.094) | 7 (0.072) | |||
| 3A | 135 (0.703) | 141 (0.727) | 0.265 | 0.606 | |
| 4A | 57 (0.297) | 53 (0.273) |
Table 2. Genotype and allele distributions of endothelin-1 Lys198Asn and rs10478694 gene polymorphisms.
Regarding rs10478694 allele comparison between patient and control group; while 48 (50%) of the patients were wild homozygous (3A/3A), 39 (40.62%) were heterozygous (3A/4A) and 9 (9.38%) were homozygous mutant (4A/4A) in the patient group, 51 (52.57%) of the patients were wild homozygous (3A/3A), 39 (40.21%) were heterozygous (3A/4A) and 7 (7.22%) were homozygous mutant (4A/4A) in the control group. There were no significant differences between the groups (p<0.845) illustrated in Figure 2.
When gene positivity of polymorphisms of all patients was compared regarding demographic characteristics, habits and backgrounds, no significant results were found. Age, gender, smoking and alcohol use, and presence of HT, DM, CAD and COPD were not associated with positivity of gene polymorphism. It was detected that there was not a significant difference in the number of patients with ET-1 gene polymorphism in ischemic CeVD patient and control groups and no correlation was found between ET-1 gene polymorphism and ischemic CeVD.
Discussion
Acute ischemic stroke is a heterogeneous disease that occurs due to several pathophysiological mechanisms. Endothelial dysfunction plays an important role in the development of CeVD. Therefore, genes that control endothelial function become important for the development of disease [16]. ET-1, produced by vascular endothelial cells, is a strong vasoconstrictor and vasopressor substance, which plays a critical role in vasoconstriction [17]. Due to being released from the damaged endothelial area and having proliferative effect on vascular smooth muscle cell, ET-1 plays an important role in vascular dysfunction in pathological conditions such as atherosclerosis, hypertension and leading cardiac hypertrophy, post-angioplasty restenosis and scleroderma [5]. In myocardial infarction, which is another disease associated with atherosclerosis and hypertension, synthesis and release of ET increase in proportion to the size and extent of infarct [18].
Endothelin-1 is considered to trigger development of CeVD and assist vascular cell growth with two receptor subtypes [19]. High-ET-1 levels have been associated with carotid atherosclerosis and asymptomatic cerebrovascular lesions [20]. Therefore, it is considered that ET-1 plays an important role in the etiology of hypertension and atherosclerosis [21].
When hypertension and atherosclerosis are considered to be major predisposing factors in the development of cerebrovascular disease, it shows that ET-1 is one of the factors that causes the development of ischemic CeVD. ET-1 plays an important role in the etiology of vasospasm developing after subarachnoid haemorrhage [22,23]. ET-1 levels in focal and global ischemia was first researched by Barone et al. [24] They measured Endothelin tissue and extracellular levels of Endothelin in ischemic rat brains and found that ET-1 levels in the ischemic cortex is significantly higher than that of nonischemic cortex 24 hours after long-term occlusion of middle cerebral artery. Levels were also found similarly high 24 hours after eighty minutes of transient occlusion and following reperfusion. Again, Estrada et al. [25] have measured the levels of plasma ET-1 in 37 patients with acute ischemic stroke and found that ET-1 levels at the immediate onset of symptoms in patients are increased approximately two-fold compared to healthy controls. The authors also reported that this increase remained high during the 7-day follow-up period.
Up-to-date evidences emphasize the importance of genetic factors as well as environmental factors [6,7]. Many phenotypes were investigated to examine the relationship between CeVD and potential endothelial genes. So far polymorphisms at ET Type A (ETa) (-231G>A, +122C>T) and ET Type B (ETb) receptor (G57S and L277L) in ET-1 gene (EDN1) (K198N) were investigated. No polymorphism or association between haplotype and CeVD was found in 300 CeVD patients [8].
Two nucleotide polymorphisms in Endothelin-1 gene (EDN1) have been reported to be associated with blood pressure. One of them is Lys198Asn polymorphism. Lys198Asn polymorphism is localized to carboxyl-terminal area from pre pro ET-1. If EDN1 Lys198Asn polymorphism plays a direct role in the ET-1 function, it can be expected to be detected in many clinical situations. Lys198Asn has been reported to play role in the development of hypertension and to affect vascular reactivity [9-12].
Endothelin-1 rs10478694 polymorphism also has functional importance. DNA studies demonstrated that rs10478694 polymorphism increases luciferase activity and trigger susceptibility to vasovagal syncope [26,27]. As rs10478694 polymorphism of EDN1 can increase ET-1 levels, it can cause a significant imbalance in vascular tone. Moreover, this polymorphism can trigger severe endothelial dysfunction when coupled with a genetic predisposition or the presence of a mutation [13-15]. However, no relationship was detected between these two polymorphisms and CeVD in our study. Also no significant differences have been detected between allele frequencies and this suggests that rs10478694 and Lys198Asn polymorphisms of EDN1 are not determinants of CeVD development.
Conclusion
In our study, we believe that ET-1 polymorphism in cerebrovascular patients is not a risk factor for CeVD and that ET-1 polymorphism does not play a role in the pathogenesis of CeVD. However, as there is plurality of different mechanisms regulating the effects of ET-1, it is understood that release of ET-1 is due to causes other than genetic factors.
Study Limitations
In this study, we aimed to investigate the relationship between ischemic cerebrovascular diseases and Lys198Asn and rs10478694 polymorphism of Endothelin-1 gene, a strong vasoconstrictor. Haven’t been studied the data of ET 1 serum levels of patients in the study and the detection for mRNA , haven't been added the data of the affecting the CeVD etiology like environmental affects, HT, including vital signs, such as atherosclerosis are limitations of our study.
References
- Sorita A, Ahmed A, Starr SR, Thompson KM, Reed DA, Dabrh AM. Off-hour presentation and outcomes in patients with acute ischemic stroke: a systematic review and meta-analysis. Eur J Intern Med 2014; 25: 394-400
- Adams HP, Biler J. Vascular diseases of the nervous system. Neurology in Clinical Practice. Elsevier (3rd edn.) 2000: 1125-1126.
- Taylor RN, Varma M, Teng NH, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J Clin Endocrinal Metabol 1990; 71: 1675-1677.
- Matsumoto H, Suzuki N, Onda H, Fujino M. Abundance ofendothelin-3 in rat intestine, pituitary gland and brain. Bio-chemBiophys Res Commun 1989; 164: 74-80.
- Gandhi CR, Berkowitz DE, Atkins WD. Endothelins. Anesthesiology 1994; 80: 892-905.
- Carmelli D, DeCarli C, Swan GE, Jack LM, Reed T, Wolf PA. Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke 1998; 29: 1177-1181.
- Jerrard-Dunne P, Cloud G, Hassan A, Markus HS. Evaluating the genetic component of ischemic stroke subtypes: a family history study. Stroke 2003; 34: 1364 -1369.
- Gormley K, Bevan S, Hassan A, Markus HS. Polymorphisms in genes of the endothelin system and cerebral small-vessel disease. Stroke 2005; 36: 1656-1660.
- Asai T, Ohkubo T, Katsuya T, Higaki J, Fu Y, Fukuda M. Endothelin-1 gene variant associates with blood pressure in obese Japanese subjects: the Ohasama Study. Hypertension 2001; 38: 1321-1324.
- Jin JJ, Nakura J, Wu Z, Yamamoto M, Abe M, Tabara Y. Association of endothelin-1 gene variant with hypertension. Hypertension 2003; 41: 163-167.
- Barden AE, Herbison CE, Beilin LJ, Michael CA, Walters BN, Van Bockxmeer FM. Association between the endothelin-1 gene Lys198Asn polymorphism blood pressure and plasma endothelin-1 levels in normal and pre-eclamptic pregnancy. J Hypertens 2001; 19: 1775-1782.
- Iglarz M, Benessiano J, Philip I, Vuillaumier-Barrot S, Lasocki S, Hvass U. Preproendothelin-1 gene polymorphism is related to a change in vascular reactivity in the human mammary artery in vitro. Hypertension 2002; 39: 209-213.
- Rajput C, Najib S, Norboo T, Afrin F, Qadar PMA. Endothelin-1 gene variants and levels associate with adaptation to hypobaric hypoxia in high-altitude natives. BiochemBiophys Res Commun 2006; 341: 1218-1224.
- Lee J, Cheong S, Kim J. Association of endothelin-1 gene polymorphisms with variant angina in Korean patients. ClinChem Lab Med 2008; 46: 1575-1580.
- Taylor MRG, Slavov D, Humphrey K, Lan Z, Cockroft J, Xiao Z. Pharmacogenetic effect of an endothelin-1 haplotype on response to bucindolol therapy in chronic heart failure. Pharmacogenet Genomics 2009; 19: 35-43.
- Hassan A,Gormley K,O'Sullivan M,Knight J,Sham P,Vallance P,Bamford J,Markus H. Endothelial nitric oxide gene haplotypes and risk of cerebral small-vessel disease. Stroke2004; 35: 654-659.
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332: 411-415.
- Luscher TF. Do we need endothelin antagonists.Cardiovasc Res 1993; 27: 2089-93.
- Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annu Rev Physiol 1999; 61: 391-415.
- Minami S, Yamano S, Yamamoto Y, Sasaki R, Nakashima T, Takaoka M. Associations of plasma endothelin concentration with carotid atherosclerosis and asymptomatic cerebrovascular lesions in patients with essential hypertension. Hypertens Res 2001; 24: 663-670.
- Schiffrin EL. Role of endothelin-1 in hypertension and vascular disease. Am J Hypertens 2001; 14: 83-89.
- Yamaji T, Johshita H, Ishibashi M, Takaku F, Ohno H, Suziki N. Endothelin family in human plasma and cerebrospinal fluid. J ClinEndocrinoMetab 1990; 71: 1611-1615.
- Suzuki R, Masaoka E, Hirata H,Marumo F, Isotani E, Hirakawa K. The role of endothelin-1 in the origin of cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg 1992; 77: 96-100.
- Barone FC,Globus MY,Price WJ,White RF,Storer BL,Feuerstein GZ. Endothelin levels ıncrease in rat focal and global ıschemia. J Cereb Blood Flow Metab 1994; 14: 337-342.
- Estrada V, Tellez MJ, Moya J, Fernandez-Durango R, Egido J, Fernandez Cruz AF. High plasma levels of endothelin-1 and atrial natriuretic peptide in patients with acute ischemic stroke. Am J Hypertens 1994; 7: 1085-1089.
- Popowski K, Sperker B, Kroemer HK, John U, Laule M, Stangl K. Functional significance of a hereditary adenine insertion variant in the 5′-UTR of the endothelin-1 gene. Pharmacogenet 2003; 13: 445-451.
- Sorrentino S, Forleo C, Iacoviello M, Guida PD, Andria V, Favale S. Endothelin system polymorphisms in tilt test-induced vasovagal syncope. ClinAuton Res 2009; 19: 347-354.