Research Article - Biomedical Research (2017) Volume 28, Issue 6
Emerge of non-albicans Candida species; evaluation of Candida species and antifungal susceptibilities according to years
Özlem Aydemir1, Tayfur Demiray1, Mehmet Köroğlu2, Yusuf Aydemir3* and Mustafa Altındiş21Department of Microbiology, Sakarya University Training and Research Hospital, Sakarya/Turkey
2Department of Microbiology, Sakarya University Faculty of Medicine, Sakarya/Turkey
3Department of Pulmonology and Intensive Care Unit, Sakarya University Faculty of Medicine, Sakarya/Turkey
- *Corresponding Author:
- Yusuf Aydemir
Department of Pulmonology
Sakarya University Education and Research Hospital, Turkey
Accepted date: November 21, 2016
Abstract
Background: Fungal infections have become frequently identified causative agents of nosocomial infections in the recent years, leading to more common use of empirical antifungal therapies. It results in increased development of drug-resistant fungal strains and resistance. In this study, we aimed to identify the Candida species isolated from various clinical samples and examine annual distribution of susceptibility to antifungals to keep a track of local resistance rates and guide treatment planning.
Methods: A total number of 169 Candida isolates, which were isolated in the samples obtained from the patients during a period of five years. Fungal identification and antifungal susceptibility tests were performed using VITEK® 2 automated system and using the mass spectrometry (Maldi-TOF).
Results: Of the strains isolated, 91 (53.5%) were identified as C. albicans, 33 (19.5%) as C. parapsilosis, 15 (8.8%) as C. glabrata, 14 (8.2%) as C. tropicalis, 5 (2.9%) as C. kefyr, 5 (2.9%) as C. famata, 2 (1.1%) as C. lipolytica, 1 (0.5%) as C. guilliermondii, and 1 (0.5%) as C. dubliniensis. Antifungal susceptibility analyses showed that there were seven strains (4.1%) resistant to each of amphotericin B, fluconazole and flucytosine, two strains resistant to voriconazole (1.1%) and one strain resistant to caspofungin (0.5%). The annual distribution of the isolation rates showed that both C. albicans and nonalbicans species became more frequently isolated over years. All drug-resistant strains were found to emerge within the past one-year period.
Conclusion: Both C. albicans and non-albicans species have become more frequently isolated within the recent years, while all of the resistant strains developed within the past one-year period. In addition to previous studies, our study results are of utmost importance, as they likely to contribute to the local monitoring of the resistance rates as well as planning or tailoring empirical therapies.
Keywords
Candida species, Candida albicans, non-albicans Candida, Fungal infections, Antifungal susceptibility.
Introduction
Fungal infections, which have become rather frequent causative agents of nosocomial infections within the recent years, have gained importance, as they are difficult-to-treat, associated with a high risk of mortality, and require long-term hospital stay. Immunosuppressive therapies, cytotoxic therapies with prolonged neutropenia, more common use of invasive catheters and wide-spectrum antibiotics, and increased numbers of patients requiring emergency care have been previously listed as the factors causing the current increase in Candida species [1,2]. While Candida albicans is still the most common causative agent of nosocomial fungal infections, some studies have reported increasing rates of species other than C. albicans. In particular, cases of Candidemia caused by the infrequent C. krusei, C. guilliermondii, and C. ciferrii species have been reported at an increasing rate over the recent years [3,4].
Gradually increasing frequency of invasive fungal infections and the widespread use of empirical antifungal therapy have resulted in the development of drug-resistant fungal strains with increased resistance rates [5]. Amphotericin B and fluconazole resistance, particularly noted in the non-albicans Candida species, obligates a review of the empirical therapies. Therefore, in vitro antifungal susceptibility tests need to be performed to select the appropriate and effective antifungal therapy [5]. Automated identification and antibiotic susceptibility systems are currently used by the majority of microbiology laboratories, providing more than 90% consistent results with liquid microdilution (LMD) which is considered to be the reference method to identify antifungal susceptibility [6]. Moreover, the introduction of recent methods such as molecular systems and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Maldi-TOF) have increased the rate of accuracy in microbiological identifications to almost 100% and shortened the time to obtain results [7-9]. The strain-level identification of the Candida species isolated as infectious agents in the hospitals and determination of their antifungal susceptibility contribute to the tracking of species and regional resistance rates, as well as to the planning of antifungal therapies. In this study, we aimed to identify the Candida species isolated from various clinical samples and examine annual distribution of susceptibility to antifungals to keep a track of local resistance rates and guide treatment planning.
Materials and Methods
A total number of 169 Candida isolates, which were isolated in the samples obtained from the patients hospitalized in different services between January 2011 and December 2015, were analysed in the Clinical Microbiology Laboratories of Ministry of Health, Sakarya University Training and Research Hospital. The same isolates with were reproduced from the same sample of individual patients were excluded from the analyses.
Suspected fungal colonies isolated from tracheal aspirates, wound, CSF, urine or blood samples were planted on Sabouraud Dextrose Agar (SDA) growth medium and incubated for 18-24 hours at 37°C. At the end of the incubation period, identification of the proliferated fungi species and antifungal susceptibility tests for amphotericin B, flucytosine, fluconazole, voriconazole and caspofungin were performed by VITEK® 2 system (Biomerieux, France), using AST-YS01 cards between 2011 and 2013, and AST-YS07 cards between 2014 and 2015. The infrequent C. famata, C. lipolytica, C. guilliermondii, C. dubliniensis, and C. ciferrii species were confirmed by the mass spectrometry method (Maldi-TOF, VITEK® MS, Biomerieux, France). The results for all of these strains were found to be consistent with the results obtained by VITEK® 2 system.
The susceptibility for amphotericin B and flucytosine was assessed based on the reference ranges specified in the Clinical Laboratory Standards Institute (CLSI) M27-A3, while susceptibility for fluconazole, voriconazole and caspofungin was determined according to the reference ranges provided for antifungal agents in the CLSI M27-S4 [10,11].
Results
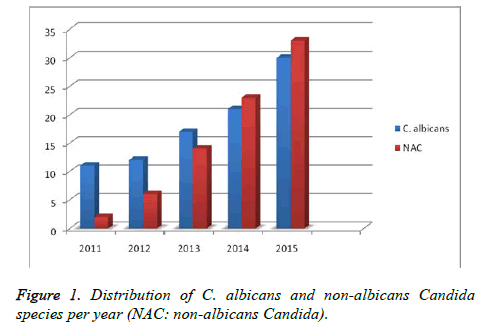
The distribution of the clinical samples included in the study was as follows: blood (n=79), wound (n=16), urine (n=51), tracheal aspirates (n=14), catheter (n=8), cerebrospinal fluid (n=1). Table 1 shows the distribution of these samples based on the services. While 91 (53.5%) of the strains isolated in this study were C. albicans, 78 (46.5%) strains were non-albicans. Among the non-albicans group, the most frequently isolated was C. parapsilosis (n=33, 42.3%) species. Table 1 shows the distribution of identified clinical samples according to the clinics and the number of C. albicans/non-albicans isolates. The number of candida isolates at species level and their susceptibility to the antifungals are presented in Table 2. During the study period, a marked increase was overall noted in the number of both albicans and non-albicans Candida isolates over the years (Figure 1).
| Clinics | Candida albicans | non-albicans candida |
|---|---|---|
| Intensive Care Unit | 56 | 60 |
| Internal Medicine | 26 | 10 |
| Oncology | 3 | 5 |
| Paediatric Surgery | 3 | 3 |
| Urology | 3 | - |
Table 1. Distribution of the Candida spp. according to the clinics.
The infrequent C. famata, C. lipolytica, C. guilliermondii, C. dubliniensis, and C. ciferrii species were confirmed by the mass spectrometry method. The results for all of these strains were found to be consistent with the results obtained by VITEK® 2 system. According to the results of antifungal susceptibility tests performed for all isolates, 7 (4.1%) strains were found to be resistant to each of amphotericin B, fluconazole and flucytosine, while 2 (1.1%) strains were resistant to voriconazole and one (0.5%) strain was resistant to caspofungin (Table 2). The annual distribution analysis of antifungal susceptibility test results showed that all resistant strains had developed within the past one-year period. Since no threshold value was recommended for C. famata species in the CLSI M27-S4 guidelines, no comments can be made on the antifungal susceptibility of these species.
| Isolate | n (%) | Antifungal drugs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amfotericin B | Fluconazole | Flucitocin | Voriconazole | Caspofungin | |||||||
| n | % | n | % | n | % | n | % | n | % | ||
| C. albicans | 91 (54) | 2 | 2.1 | 1 | 1.1 | 2 | 2.1 | 1 | 1 | 0 | 0 |
| C. parapsilosis | 33 (19.5) | 2 | 6.1 | 2 | 6.1 | 2 | 6.1 | 0 | 0 | 1 | 3.03 |
| C. tropicalis | 14 (8.2) | 2 | 14.2 | 2 | 14.2 | 1 | 7.1 | 0 | 0 | 0 | 0 |
| C. kefyr | 5 (2.9) | 0 | 0 | 0 | 0 | 1 | 20 | 0 | 0 | 0 | 0 |
| C. lipolytica | 2 (1.1) | 1 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. glabrata | 15 (8.8) | 0 | 0 | 2 | 13.3 | 1 | 6.6 | 1 | 6.6 | 0 | 0 |
| C. guillermondii | 1 (0.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. famata | 5 (2.9) | - | - | - | - | - | - | - | - | - | - |
| C. dubliniensis | 1 (0.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. ciferii | 2 (1.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| Total | 169 | 7 | 4.1 | 7 | 4.1 | 7 | 4.1 | 2 | 1.1 | 1 | 0.5 |
Table 2. Resistance rates of Candida isolates against antifungal drugs.
Discussion
Nosocomial fungal infections have increasingly become an important cause of the increased morbidity and mortality and the leading one of these infections involve fungemia due to Candida species [12,13]. Along with the increased rates of infections caused by Candida species, the diversity of the species causing such infections has also changed. While C. albicans is the leading cause of nosocomial Candida infections, the infections caused by non-albicans species such as C. tropicalis, C. lusitaniae, C. krusei, C. parapsilosis, and C. glabrata have also increasingly become more common [14].
In the Artemis Global antifungal susceptibility follow-up study, a total number of 205.329 yeast samples were collected at 134 centers between the years 1997 and 2005. Among the isolated yeast fungi, almost 95.7% were found to be Candida species and C. albicans was identified at a rate of 65.6%. When the 1997 and 2000 and 2001 and 2005 periods were compared, C. albicans frequency was found to decrease from 70.9% to 63.5%, while significant increases were recorded for C. glabrata (from 10.2% to 11.4%), C. tropicalis (from 5.4% to 7.5%), and C. parapsilosis (from 4.8% to 6.6%) species. These findings support the increased number of the non-albicans species [15]. In the present study, the evaluation of the annual distribution of Candida species showed a significant increase in the frequencies of both albicans and non-albicans Candida infections over years (Figure 1).
In the studies investigating the distribution of Candida species in Turkey, the most frequently isolated Candida strain in the clinical samples was C. albicans. Among the non-albicans species, the most commonly isolated strains are C. parapsilosis, C. tropicalis, and C. glabrata [16-19]. The results obtained from the present study as well as the other studies performed in the recent years highlight the year by year increasing importance of non-albicans Candida species [18-20].
Delayed diagnosis may also result in increased mortality rates of invasive fungal infections. Therefore, methods which provide strain-level identification of the Candida species in a rapid and reliable manner are required to initiate the antifungal therapy. Since identification of the Candida species by conventional methods takes a long time and requires experience as well as manpower, the laboratories currently prefer utilizing methods such as VITEK® 2 automated system, and API 20C AUX (Biomerieux, France) which offer rapid identification. Several previous studies reported that the results of VITEK® 2 automated system, which we have also used in the present study, are 92-95% consistent with the results obtained by conventional methods [7,21-23].
Rapidly and accurately provided antifungal susceptibility test results are expected to contribute to the treatment planning, while reducing mortality. Since 1997, the liquid microdilution (LMD) method has been used as the commonly accepted reference method to test susceptibility of Candida isolates to amphotericin B, flucytosine, fluconazole and itraconazole, and voriconazole was also added to this list in 2002. However, the results of this method are obtained only in a long time and it imposes a high workload. Tests which spectrophotometrically identify Candida proliferation have been developed in the recent years and the availability of systems such as VITEK® 2 offers an opportunity to perform fully-automated antifungal susceptibility studies [6]. The results of VITEK® 2 automated system, which we have also used in the present study, are known to be more than 90% consistent with the result obtained by the reference LMD method [6].
Amphotericin B resistance is a rare entity with varying rates between 0-7 and 7% in the previous studies on amphotericin B resistance in Candida isolates [12,19,24-26]. In the present study, 4.1% of the Candida strains were found to be resistant to amphotericin B. Two (2.1%) of the C. albicans isolates, and two for each of the non-albicans C. parapsilosis, and C. tropicalis isolates (6.1-14.2%) were found to be resistant to amphotericin B.
In the previous studies, the rate of fluconazole resistance in Candida isolates varied between 0 and 7% [14,16,27,28]. Consistent with these findings, the rate of fluconazole resistance in the present study was 4.1%. When the distribution of fluconazole resistance between different species was evaluated, one (1.1%) of the C. albicans isolates was resistant and among the non-albicans species, two isolates were resistant in each of the C. parapsilosis (6.1%), C. tropicalis (14.2%), and C. glabrata (13.3%) species. Studies performed in Turkey and worldwide have established that fluconazole resistance shows regional variability, varying between 0 and 25% [15,16,29-32]. On the other hand, the rate of fluconazole resistance was as high as 13.3% in C. glabrata isolates in the present study. This can be attributed to the regional differences and widespread use of fluconazole as an empirical antifungal therapy.
Among the recent studies performed by voriconazole, a widespectrum antifungal drug, some were unable to identify any case of voriconazole resistance, while some reported a resistance rate of 4% in C. albicans, 1% in C. parapsilosis, and 1.8% in C. glabrata species [19,33]. Wang et al. previously reported voriconazole resistance in C. albicans (0.7%), C. glabrata (17.8%), C. krusei (11.1%), C. lipolytica (90%), and C. tropicalis (5.7%) strains [34]. In another study, Odds et al. reported voriconazole resistance at a rate of 4.5% in C. glabrata species [35]. In the present study, voriconazole resistance was found in 1.1% of all strains (one in each of C. albicans and C. glabrata). Based on these resistance rates, voriconazole currently appears to be the safest and the most useful agent among azole derivatives.
Caspofungin and the other echinocandins are the agents which are usually preferred, when the patients fail to response to other antifungals [36-38]. In the present study, caspofungin resistance was detected only in one C. parapsilosis isolate. Among the other studies performed in Turkey, Yenisehirli et al. did not identify caspofungin resistance in any of the Candida strains and reported moderate susceptibility in 15% [39]. Caspofungin susceptibility rates previously reported by the similar studies varied between 98 and 100% [32,33,38].
For the Candida species, several studies reported rates of flucytosine resistance at a range of 0% to 9.05% [33,40]. Flucytosine resistance was identified in C. albicans (2.4%), C. glabrata (0%), C. parapsilosis (0.5%), and C. tropicalis (10.3%) isolates [40]. In the present study, flucytosine resistance was detected at a rate of 4.1% (2 C. albicans, 2 C. parapsilosis, 1 C. tropicalis, 1 C. glabrata, 1 C. kefyr).
In the present study, due to limited resources, not all isolates were able to be identified using the mass spectrometry method and analysed by broth dilution in terms of antifungal susceptibility.
In conclusion, species-level identification and antifungal susceptibility assessment of Candida strains should be obviously performed to ensure treatment success, shorten the duration of hospital stay, and reduce the mortality rates in cases with Candida infections. Our study results suggest that the number of all Candida strains and, in particular, the number of non-albicans isolates, have increased over years. All drugresistant strains were found to emerge within the past one-year period. In addition to previous studies, our study results are of utmost importance, as they likely to contribute to the local monitoring of the resistance rates as well as planning or tailoring empirical therapies.
References
- Yang YL, Lo HJ. Mechanisms of antifungal agent resistance. J Microbiol Immunol Infect 2001; 34: 79-86.
- Cheng MF, Yu KW, Tang RB, Fan YH, Yang YL, Hsieh KS, Ho M, Lo HJ. Distribution and antifungal susceptibility of Candida species causing candidemia from 1996 to 1999. Diagn Microbiol Infect Dis 2004; 48: 33-37
- Pfaller, M A, Diekema, DJ, Messer SA, Boyken L, Hollis RJ, Jones RN. In vitro activities of voriconazole, posaconazole, and four licensed systemic antifungal agents against Candida species infrequently isolated from blood. J Clin Microbiol 2003; 41: 78-83.
- Demiray T, Hafizoglu T, Koroglu M, Özbek A, Altindis M. The first case of Stephanoascus ciferrii infection in a new born and review of literature. Nobel Medicus Journal 2015; 11: 97-100.
- Pfaller MA, Diekema D J. Progress in antifungal susceptibility testing of candida spp. by use of clinical and laboratory standards institute broth microdilution methods, 2010 to 2012. J Clin Microbiol 2012; 50: 2846–2856.
- Pfaller MA., Diekema DJ, Procop GW, Rinaldi MG. Multicenter comparison of the VITEK2 Yeast Susceptibility Test with the CLSI Broth Microdilution Reference Method for testing fluconazole against Candida spp. J Clin Microbiol 2007; 45: 796-802.
- Yaman G, Akyar I, Can S. Evaluation of the MALDI TOF-MS method for identification of Candida strains isolated from blood cultures. Diagn Microbiol Infect Dis 2012; 73: 65-67.
- Putignani L, Del Chierico F, Onori M, Mancinelli L, Argentieri M, Bernaschi P, Coltella L, Lucignano B, Pansani L, Ranno S, Russo C, Urbani A, Federici G, Menichella D. MALDI-TOF mass spectrometry proteomic phenotyping of clinically relevant fungi. Mol Biosyst 2011; 7: 620-629.
- Pulcrano G, Iula DV, Vollaro A, Tucci A, Cerullo M, Esposito M, Rossano F, Catania MR. Rapid and reliable MALDI-TOF mass spectrometry identification of Candida non-albicans isolates from bloodstream infections. J Microbiol Methods 2013; 94: 262-266.
- Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement. Wayne: Clinical and Laboratory Standards Institute; 2012 (Document M27-S4).
- Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts. 3rd ed. Wayne: Clinical and Laboratory Standards Institute; 2008 (Approved standard. M27-S3).
- Hamid SUB, Tan S, Ridzuan SNA, Seman MSC, Ramli R, Khaithir TMN. Antifungal susceptibility patterns among Candida species isolated from blood at Universiti Kebangsaan Malaysia Medical Centre. Sains Malaysiana 2012; 41: 961-967.
- Akçam EA. The Analysis of Candidemia Cases Followed in The Cukurova University Hospital According to Their Epidemiologic, Clinical and Antifungal Susceptibility Characteristics. Çukurova Üniversitesi, 2009.
- Gültekin B, Eyigör M, Telli M. A Retrospective investigation of Candida species isolated from blood cultures during a seven-year period. Ankem J 2010; 24: 202-208.
- Pfaller MA, Diekema DJ, Gibbs DL. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2005: an 8.5-year analysis of susceptibilities of Candida species and other yeast Species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol 2007; 45: 1735-1745.
- Erdem F, Tuncer EG, Oral B, Karakoç E, Demiröz AP, Tülek N. Epidemiological and Microbiological Evaluation of Nosocomial Infections Caused by Candida Species. Mikrobiyol Bul 2012; 46: 637-648.
- Satilmis ÖK, Akkaya Y, Ergin Ç, Kaleli I. Slime factor production in isolates of Candida spp origin from various clinical specimens. Pamukkale Med J 2011; 4: 25-29.
- Sarigüzel FM, Koç AN, Karagöz S. Distribution and Antifungal Susceptibilities with VITEK 2 system of Yeast Isolated from Blood Cultures. J Harran University Med Faculty 2015; 12.
- Etiz P, Kibar F, Ekenoglu Y, Yaman A. Retrospective Evaluation of Distribution and Antifungal Susceptibilities of Candida Species Isolated from Blood Cultures. Ankem J 2015; 29:105-113.
- Zer Y, Balci I. identification and antifungal susceptibility of Candida strains isolated from patients in intensive care unit. J Turkish Soc Microbiol 2002; 32: 230-234.
- Karabiçak N, Altun HU, Karatuna O, Hazırolan G, Aksu N, Adiloğlu A, Akyar I. Evaluation of common commercial systems for the Identification of yeast isolates in microbiology laboratories: A Multicenter Study. Mikrobiyol Bul 2015; 49: 210-220.
- Meletiadis J, Arabatzis M, Bompola M, Tsiveriotis K, Hini S, Petinaki E, Velegraki A, Zerva L. Comparative evaluation of three commercial identifi cation systems using common and rare bloodstream yeast isolates. J Clin Microbiol 2011; 49: 2722-2727.
- Hata DJ, Hall L, Fothergill AW, Larone DH, Wengenack NL. Multicenter evaluation of the new VITEK 2 advanced colorimetric yeast identification card. J Clin Microbiol 2007; 45: 1087-1092.
- Yenisehirli G, Bulut Y, Günday E. Antifungal Susceptibility of Candida albicans Isolates Recovered from Blood Cultures of Intensive Care Unit Patients. Ankem J 2007; 21: 146-149.
- Bayram Y, Gültepe B, Özlük S, Güdücüoglu H. Investigation and Identification of the Antifungal Susceptibility of Candida Origin Isolated from Various Clinical Samples. Van Med J 2012; 19: 177-181.
- Mokaddas EM, Al-Sweih NA, Khan ZU. Species distribution and antifungal susceptibility of Candida bloodstream isolates in Kuwait: a 10-year study. J Med Microbiol 2007; 56: 255-259.
- Altuncu E, Bilgen H, CerikçioÄŸlu N, Ilki A, Ulger N, Bakır M, Akman I, Ozek E. Neonatal Candida infections and the antifungal susceptibilities of the related Candida species. Mikrobiyol Bul 2010; 44: 593-603.
- Kuzucu Ç, Yetkin G, Çaliskan A. Antifungal susceptibilities and subtype identifications of Candida species, isolated in blood cultures throughout one-year period. Erciyes Med J 2007; 29: 115-19.
- Diekema DJ, Messer SA, Brueggemann AB, Coffman SL, Doern GV, Herwaldt LA, Pfaller MA. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J Clin Microbiol 2002; 40: 1298-1302.
- Yüksekkaya S, Findik D, Arslan U. Molecular epidemiology and antifungal susceptibility Of Candida Species Isolated from urine samples of patients in intensive care unit. Mikrobiyol Bul 2011; 45: 137-149.
- Aydin F, Bayramoglu G, Guler NC, Kaklikkaya N, Tosun I. Bloodstream yeast infections in a university hospital in Northeast Turkey: a 4-year survey. Med Mycol 2011; 49: 316-319.
- Saracli MA, Gumral R, Gul HC, Gonlum A, Yildiran ST. Species distribution and in vitro susceptibility of Candida blood stream isolates to six new and current antifungal agents in a Turkish tertiary care military hospital, recovered through 2001 and 2006. Mil Med 2009; 174: 860-5.
- Hazirolan G, Yildiran D, Baran I, Mumcuoğlu I, Aksu N. Evaluation of species distribution and antifungal susceptibility profiles of Candida isolates from hospitalized patients. J Turkish Hygiene Exp Biol 2015; 72: 17-26.
- Wang H, Xiao M, Chen SC, Kong F, Sun ZY, Liao K, Lu J, Shao HF, Yan Y, Fan H, Hu ZD, Chu YZ, Hu TS, Ni YX, Zou GL, Xu YC. Invitro susceptibilities of yeast species to fluconazole and voriconazole as determined by the 2010 National China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study. J Clin Microbiol 2012; 50: 3952-3959.
- Odds FC, Hanson MF, Davidson AD, Jacobsen MD, Wright P, Whyte JA, Gow NA, Jones BL. One year prospective survey of Candida bloodstream infections in Scotland. J Med Microbiol 2007; 56: 1066-1075.
- Nelson PW, Lozano-Chiu M, Rex J.H. Invitro growth-inhibitory activity of pneumocandins L-733,560 and L-743,872 against putatively amphotericin B and fluconazole-resistant Candida isolates: influence of assay conditions. J Med Vet Mycol 1997; 35: 285-287.
- Messer SA, Diekema DJ, Boyken L, Tendolkar S, Hollis RJ, Pfaller MA. Activities of micafungin against 315 invasive clinical isolates of fluconazole-resistant Candida spp. J Clin Microbiol 2006; 44: 324-326.
- Rogers TR, Johnson EM, Munro C. Echinocandin antifungal drug resistance J Invasive Fungal Infect 2007: 199-205.
- Yenisehirli G, Bulut N, Yenisehirli A, Bulut Y. In Vitro Susceptibilities of Candida albicans Isolates to Antifungal Agents in Tokat, Turkey. Jundishapur J Microbiol 2015; 8: e28057.
- Messer SA, Jones RN, Moet GJ, Kirby JT, Castanheira M. Potency of anidulafungin compared to nine other antifungal agents tested against Candida spp., Cryptococcus spp., and Aspergillus spp. Results from the global SENTRY Antimicrobial Surveillance Program. J Clin Microbiol 2010; 48: 2984-2987.