Research Article - Biomedical Research (2018) Volume 29, Issue 5
Emblica total phenolic acids for treatment of acetaminophen-induced liver injury in mice
Hongying Wang1, Yan Xue1, Xiaoli Shi2 and Fei Wang3*
1Liaoning Grand Nuokang Biopharmaceutical Co. Ltd, Shenyang of Liaoning Province, PR China
2Pharmacy Department of Affiliated Zhongshan Hospital of Dalian University, Dalian, PR China
3College of Pharmacy, Liaoning University of Traditional Chinese Medicine, Dalian, PR China
- *Corresponding Author:
- Fei Wang
College of Pharmacy
Liaoning University of Traditional Chinese Medicine, PR China
Accepted on November 27, 2017
Abstract
To investigate the protective effect of Emblica Total Phenolic Acids (ETPA) on acute liver injury in mice and its possible mechanism. Mouse model of acute liver injury was established, which was induced by Acetaminophen (APAP). The mice were divided into normal group, model group and ETPA groups (15, 30 and 60 mg•kg-1). UV-Vis spectrophotometry was used to detect serum Alanine Aminotransferase (ALT) and Aspartate Amino Transferase (AST) activities of various groups, as well as Superoxide Dismutase (SOD) and Malonaldehyde (MDA) levels in liver homogenates. HE staining was performed to observe the pathological changes in liver. Compared to the model group, intragastric administration of ETPA (30 and 60 mg•kg-1) could significantly decrease the elevated activities of ALT and AST in serum. ETPA was also found to decrease the elevated MDA and SOD levels in liver homogenates. ETPA could significantly improve the degree and extent of hepatocellular necrosis and reduce inflammatory cell infiltration. ETPA has a protective effect on APAP-induced acute liver injury in mice, and relevant mechanism may be associated with its anti-oxidation effect.
Keywords
Emblica total phenolic acids, Acetaminophen, Acute liver injury, Anti-oxidation.
Introduction
Liver injury is a disease of liver dysfunction caused by a variety of pathogenic factors including viral infection, radiation injury, excessive intake of ethanol and food additives, toxic food intake, etc. [1]. Clinically, liver injury is manifested primarily as apparent medical history within 72 h, abnormal elevation of liver function indices in short-term, jaundice, etc. [2]. Acute liver injury is the initial link and common pathway for occurrence and development of multiple liver diseases, as well as for eventual liver failure [3]. At present, acute liver injury is generally considered a reversible disease, but there is no desirable drug yet clinically. Extensive studies have shown that natural medicine and traditional Chinese medicine have reliable efficacy and small adverse reactions in the treatment of acute liver injury. In recent years, the incidence of druginduced liver injury has been rising with the continuous introduction of new drugs. According to domestic and foreign reports, more than 1,000 kinds of drugs can cause druginduced liver injury [4,5]. Chinese medicine Yuganzi is the dried ripe fruits of Phyllanthus emblica L. in the genus Phyllanthus of the family Euphorbiaceae. Emblica enters the lung and stomach meridians, which is sweet, acid, astringent and cool. With heat-clearing, blood-cooling, digestionpromoting, stomach-invigorating, saliva-producing and coughsuppressing functions, it is clinically used for the treatment of blood heat, blood stasis, indigestion, abdominal distension, cough, sore throat, dry mouth, etc. [6]. Active constituents of emblica are polyphenols, which are classified into flavonoids and phenolic acids [7-11]. Emblica has a significant protective effect on DNA damage. Besides, the existing literature has proved that it has a strong anti-oxidation effect. As a promising antioxidant regulator, emblica is worth further verification and development [12-14]. Furthermore, research has shown that ETPA has a definite efficacy for liver cancer, but its inhibitory role in liver injury has never been reported as far as the authors know. This paper studies the protective effect of ETPA on APAP-induced liver injury by establishing a mouse model of APAP-induced acute liver injury.
Materials and Methods
Chemical reagents
APAP (batch No.: A7302-100G, size: 100 g/bottle, purity: 98%, Sigma, USA); ETPA (purity: 98.1%, self-prepared); bifendate (Bif, batch No.: A02140712, size: 1.5 mg/pill, Zhejiang WEPON Pharmaceutical Co., Ltd.); ALT, AST, SOD and MDA kits (purchased from Nanjing Jiancheng Bioengineering Institute).
Main instruments
8455 dual-wavelength UV-Vis spectrophotometer (Agilent, USA); JA2003A electronic analytical balance (Shanghai Jingtian Electronic Instrument Factory); paraffin slicing machine (Leica, Germany); baking machine, incubator (Shanghai Yiheng Instruments Co., Ltd.); IX 71 inverted fluorescence microscope (Olympus, Japan); Image-Pro Plus image analysis system (Media Cybernetics); 30 K lowtemperature high-speed centrifuge (Sigma, USA); SIM F140 ice maker (Sanyo, Japan); UPT II 10 T water purification system (Wuhan ULUPURE Purification Equipment Co., Ltd.); -80°C ultralow temperature freezer (Qingdao Haier BioMedical Co., Ltd.).
Animals
Male SPF Kunming mice, weighing 18-22 g, aged 5-6 w, with a certification No. of SCXK (Wan) 2011-002, were provided by the Laboratory Animal Center of Anhui Medical University. The mice were adaptively fed for 3 d, then fed and watered ad libitum. Alternate 12 h periods of light and darkness were maintained daily. Temperature was maintained between 20-25°C, while relative humidity was (45-65%). This study was approved by the Laboratory Animal Center and Ethics Committee of Anhui Medical University.
Animal grouping and modeling
The animals were randomly divided into 6 groups: normal control group, model group, ETPA groups (15, 30 and 60 mg•kg-1) and positive control group (Bif, 150 mg•kg-1) (n=8 in each group). Mice were intragastrically administered corresponding dosage (0.2 ml/10 g) of ETPA or Bif daily. The normal and model groups were intragastrically administered an equal volume of 0.9% NaCl solution. On d 8, all mice were fasted for 10 h after the last administration. APAP (300 mg•kg-1) was given by intraperitoneal injection, while the normal group was intraperitoneally injected an equal volume of 0.9% NaCl solution. After an additional 12 h of fasting, blood samples were taken from the orbital venous plexus and centrifuged at 3000 r•min-1 for 20 min (4°C), then supernatant was aspirated for biochemical marker detection. The mice were sacrificed by cervical dislocation, and the same parts of livers were taken for the determination of MDA and SOD levels, while the remaining livers were fixed in 10% formaldehyde for histopathological examination.
Serum ALT, AST detection
The activities of ALT and AST in serum were determined by Reitman-Frankel method as per the kit instructions.
Liver tissue SOD, MDA detection
Liver tissues of mice were added to the homogenizer according to tissue: 0.9% NaCl solution (1:9) and ground on ice to prepare into 10% liver homogenates, followed by operation according to the kit instructions.
Liver, spleen and thymus index
The mice were killed after taking blood, the quality of liver, spleen and thymus was weighed. Liver index, spleen index and thymus index were calculated by m/g unit.
Liver index=liver wet weight × 1000/body weight (unit G)
Spleen index=spleen wet weight × 1000/body weight (unit G)
Thymus index=thymus wet weight × 1000/body weight (unit G)
Pathological examination of liver tissue in mice
Left liver lobe tissues were fixed in 10% formaldehyde, dehydrated with ethanol, cleared in xylene, embedded in paraffin, serially sectioned at 5 μm and routinely stained with HE. After mounting with neutral resin, liver histopathological changes were observed under light microscope.
Statistical analysis
The experimental data were analysed using SPSS 17.0 statistical software. Intergroup comparison was performed by one-way ANOVA, while pairwise comparison was done by Dunnet's test. The results were expressed as x̄ ± s.
Results
Effects of ETPA on serum ALT, AST in mice with APAP-induced acute liver injury
Compared to the normal group, the serum activities of ALT and AST for mice in APAP model group were significantly elevated. Compared to the model group, ETPA (30, 60 mg•kg-1) and Bif (150 mg•kg-1) could all significantly reduce the APAP-induced elevated activities of ALT and AST in mice (Table 1).
| Group | Dose/mg•kg-1 | ALT/U•L-1 | AST/U•L-1 |
|---|---|---|---|
| Normal group | - | 40.11 | 73.24 |
| Model group | - | 87.89** | 179.2** |
| ETPA group | 15 | 77.58 | 161.7 |
| 30 | 69.42# | 138.64# | |
| 60 | 62.35## | 120.55## | |
| Bif group | 150 | 48.64## | 111.24## |
Comparison with the normal group, **P<0.001; comparison with the model group, #P<0.05, ##P<0.01
Table 1. Effects of ETPA on serum ALP and AST in mice (n=8).
Effects of ETPA on serum SOD, MDA in mice with APAP-induced acute liver injury
Compared to the normal group, the serum SOD activity decreased while serum MDA level increased markedly for mice in the APAP model group. Compared to the model group, ETPA (30, 60 mg•kg-1) and Bif (150 mg•kg-1) could all significantly enhance SOD activity and lower MDA level in mice (Table 2).
| Group | Dose/mg•kg-1 | SOD/U•mgprot-1 | MDA/U•mgprot-1 |
|---|---|---|---|
| Normal group | - | 156.34 | 0.74 |
| Model group | - | 112.1** | 1.19** |
| ETPA group | 15 | 132.85 | 1.01 |
| 30 | 140.73# | 0.69# | |
| 60 | 145.18## | 0.81## | |
| Bif group | 150 | 144.52## | 0.79## |
Comparison with the normal group, **P<0.001; comparison with the model group, #P<0.05, ##P<0.01
Table 2. Effects of ETPA on serum SOD and MDA in mice (n=8).
Effects of ETPA on liver, spleen and thymus indices of mice with APAP-induced acute liver injury
Compared to the normal group, the liver and spleen indices of mice in the APAP model group were all markedly increased, while the thymus index showed no obvious changes. Compared to the model group, ETPA (60 mg•kg-1) and Bif (150 mg•kg-1) could both markedly reduce the APAP-induced elevated liver index of mice (Table 3).
| Group | Dose/mg•kg-1 | Liver/g | Spleen/g | Thymus/g |
|---|---|---|---|---|
| Normal group | - | 60.1 ± 3.25 | 3.8 ± 0.99 | 3.8 ± 0.36 |
| Model group | - | 70.4 ± 2.1* | 5.5 ± 0.64* | 3.6 ± 0.73 |
| ETPA group | 15 | 64.9 ± 1.4 | 4.9 ± 0.52 | 3.9 ± 0.69 |
| 30 | 62.8 ± 3.23 | 4.9 ± 0.79 | 3.7 ± 0.85 | |
| 60 | 59.3 ± 2.95# | 4.7 ± 0.37 | 3.4 ± 0.59 | |
| Bif group | 150 | 58.1 ± 3.73# | 4.5 ± 0.88 | 3.6 ± 0.77 |
Comparison with the normal group, *P<0.05; comparison with the model group, #P<0.05
Table 3. Effects of ETPA on serum liver, spleen and thymus indices of mice (n=8).
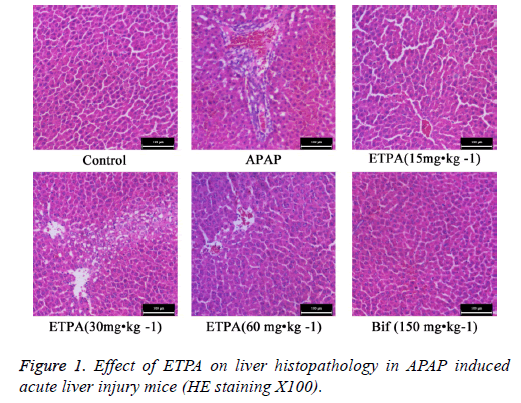
Effects of ETPA on liver histopathology of mice with APAP-induced acute liver injury
HE staining revealed that the hepatic lobules in liver tissues of normal mice had clear structure, and the liver cords were arranged orderly from the central veins to the surroundings. Comparatively, APAP model mice exhibited abnormal hepatic lobular structure, disorderly liver cord structure, and obvious interstitial infiltration of inflammatory cells. ETPA (15, 30 and 60 mg•kg-1) and Bif (150 mg•kg-1) could all markedly attenuate the APAP-induced inflammatory cell infiltration and hepatic cord disturbance (Figure 1).
Discussion
Liver, as the main organ for detoxifying drugs and toxins, is highly vulnerable to the effects of harmful factors to cause liver injury [15]. Liver injury is a recoverable disease. In the early stage of liver injury, the damaged liver cells can regain normal function through drug therapy. But if the treatment is not timely, the damaged liver cells will not recover and may develop into liver fibrosis, liver cirrhosis and even liver cancer. Therefore, the researches on pathogenesis of liver injury and drug intervention effect are of great significance to the prevention and treatment of chronic liver diseases. ETPA is a kind of glycosides with multiple pharmacological activities including anti-oxidative and anti-free radical formation effects, which is isolated from the dried ripe fruits of Phyllanthus emblica L. In this paper, the protective effect of ETPA on APAP mice is investigated through mouse model of APAPinduced acute liver injury.
APAP-induced mouse acute liver injury model is a commonly used animal model of liver injury at present, which is an ideal model for screening hepatoprotective drugs with its fast modeling, easy replication and good stability [16]. After entering the body, 90% of APAP bonds with sulfate and glucuronic acid under the catalysis of sulfotransferase and UDP-glucuronosyltransferase to form non-toxic substances and are discharged from the urine. Approximately 5% of APAP, which is not involved in biotransformation, is excreted from the initial urine in the original form. The remaining is metabolized by the cytochrome P450 enzyme system (mainly CYP1A2 and CYP2E1) in the hepatocyte endoplasmic reticulum. In the presence of NADPH, APAP can be transformed into hepatotoxic substance N-acetyl-pbenzoquinone imine (NAPQI), and GSH can bond with it to relieve adverse liver reactions. When APAP is overdosed, a large amount of sulfotransferase and UDPglucuronosyltransferase are consumed, which leads to excessive concentration of NAPQI in hepatocytes to bond with enzymatic and non-enzymatic systems in the liver, thereby forming hepatotoxic products to result in hepatocellular injury [17]. Meanwhile, substantial free radicals are produced in the mouse body to cause lipid peroxidation, which leads to decreased SOD activity and increased MDA production [18]. It has been reported that during acute liver injury, liver cell membrane permeability increases to result in substantial release of ALT, AST and marked elevation of blood AST, ALT levels [19]. The increase of free radicals and the consumption of antioxidant enzymes can cause oxidative stress reaction, thereby inducing liver injury. Therefore, the blood ALT, AST levels and liver homogenate SOD, MDA activities are important indicators for evaluating the severity of liver injury. In the present study, we examined the effects of ETPA on the ALT, AST levels in blood and the SOD and MDA activities in liver tissues of mice. The results showed that ETPA significantly decreased AST and ALT levels, increased SOD activity in liver homogenates, and decreased MDA level. Liver pathology also revealed that the degree and extent of hepatocellular necrosis and inflammatory cell infiltration were all markedly reduced compared to the model group. This suggests that ETPA can improve APAP-induced oxidative damage of hepatocytes to a certain extent.
In conclusion, ETPA has a certain protective effect on APAPinduced liver injury, and relevant mechanism may be associated with reduced free radical production and increased activity of antioxidant enzymes.
References
- Negoi I, Paun S, Stoica B, Tanase I, Vartic M, Negoi RI, Hostiuc S, Beuran M. Latest progress of research on acute abdominal injuries. Mod J Integr Trad Chinese West Med 2009; 18: 2211-2213.
- Liu B, Jiang SM. The clinical observation of traditional Chinese medicine on acute liver injury. J Liaoning Univ Trad Chinese Med 2014; 16: 101-103.
- Zhou Q, Liu FP, Liu YS, Zhao YL, Li CW, Li R, Zhang XY. Research on animal model building method that carbon tetrachloride causes acute liver injury of mice. J Northeast Agr Univ 2012; 43: 77-81.
- Letse MD, Poteru C, Talwalkar JA. Drug-induced liver injury. Nippon Yakuriga ku Iasshi 2006; 127: 454-459.
- Weiler-Normann C, Schramm C. Drug induced liver injury and its relationship to autoimmune hepatitis. J Hepatol 2011; 55: 747-749.
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the Peoples Republic of China 2010. Beijing: China Med Sci Press 2010; 179: 187.
- Liu XL, Cui C, Zhao MM, Wang JS, Luo W, Yang B, Jiang YM. Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chem 2008; 109: 909-915.
- Habib-ur R, Yasin KA, Choudhary MA, Khaliq N, Atta-ur R, Choudhary MI, Malik S. Studies on the chemical constituents of Phyllanthus emblica. Nat Prod Res 2007; 21: 775-781.
- Liang CY, Zhen HS, Tong XL. Studies on the chemical constituents from leaves of Phyllanthus emblica. Chinese Trad Pat Med 2009; 31: 761-763.
- El-Desouky SK, Young RS, Kimi YK. A newcytotoxic acylated apigenin glucoside from Phyllanthus emblica L. Nat Prod Res 2008; 22: 91-95.
- Zhang YJ, Abe T, Tanaka T. Two new acylated flavanone glycosides from the leaves and branches of Phyllanthus emblica. Chem Pharm Bull (Tokyo) 2002; 50: 841-843.
- Li P, Yang ZT, Peng BC, Zhen DD. Anti-immunological-hepatic fibrosis effect of Phyllanthus emblica L. on rat (I). Chinese J Exp Trad Med Formul 2010; 16: 171-173.
- Zhu W, Yu HB, Dai C. Inhibition effect research on liver injury and inflammation of nonalcoholic fatty liver disease of rat. J Med Res 2012; 41: 140-143.
- Pramyothin P, Samosorn P, Poungshompoo S, Chaichantipyuth C. The protective effects of Phyllanthus emblica Linn. extract on ethanol induced rat hepatic injury. J Ethnopharmacol 2006; 107: 361- 364.
- Dönmez M, Uysal B, Poyrazoglu Y, Oztas YE, Türker T, Kaldirim U, Korkmaz A. PARP inhibition prevents acetaminophen-induced liver injury and increases survival rate in rats. Turk J Med Sci 2015; 45: 18-26.
- Zhang Q, Chen H, Peng SL, Bai Y, Zhong XH. Progress of animal model preparation study on acute liver injury. J Jilin Med Coll 2011; 32: 216-220.
- Bu XJ. Research progress on acute liver injury animal model making. China Med Herald 2014; 11: 166-168.
- Pan HZ, Li H, Chen WH, Qiu XH, Na LX. The protection effect of lycopene on DEN injures rats liver. J Toxicol 2006; 20: 104-105.
- He W, Song SS, Yuan PF, Lu JT, Wei W. The therapeutic effect and mechanism of Tripterine on curing rats liver fibrosis caused by DEN. Chinese Pharmacol Bull 2013; 29: 519-524.