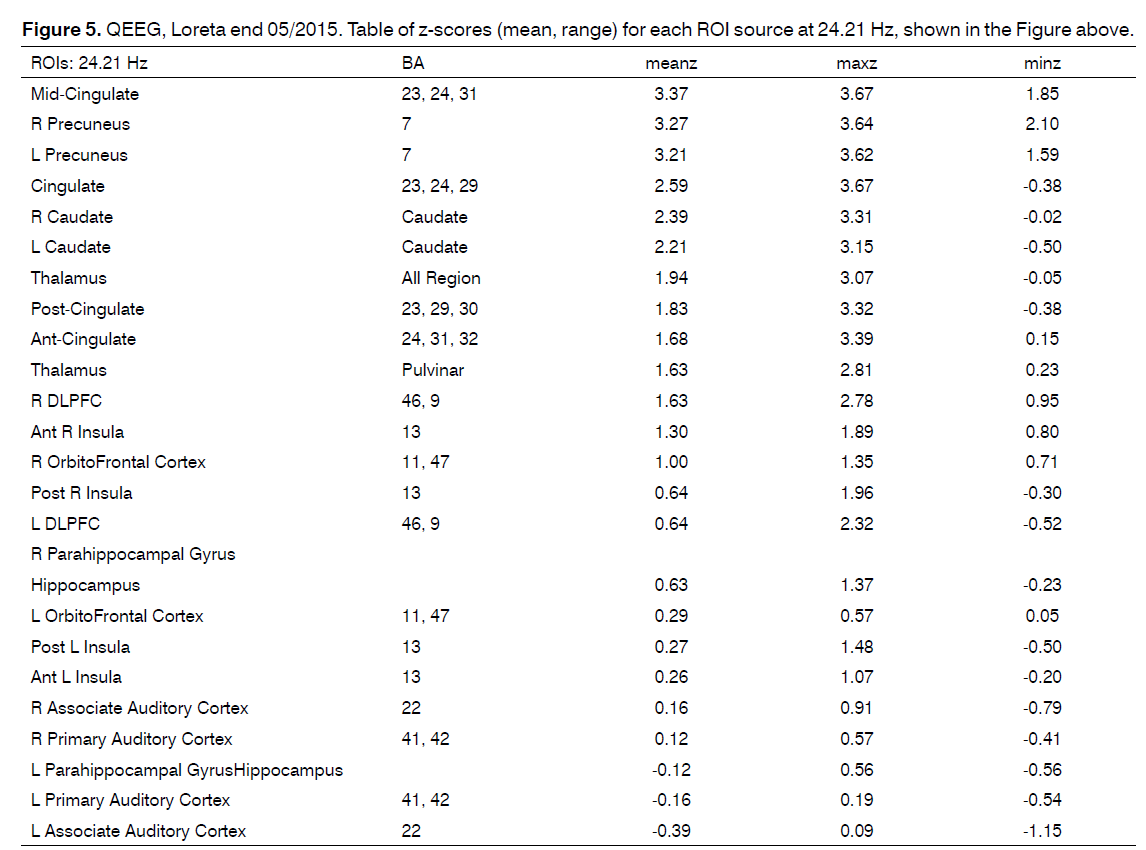Research Article - The International Tinnitus Journal (2014) Volume 19, Issue 1
Electrophysiology quantitative electroencephalography/low resolution brain electromagnetic tomography functional brain imaging (QEEG LORETA): Case report: Subjective idiopathic tinnitus - predominantly central type severe disabling tinnitus
1M.D., F.A.C.S. Prof. Emeritus Clinical Otolaryngology.
2PhD Assistant Clinical Professor, Department Otolaryngology, (Retired). SUNY/Downstate 450 Clarkson Ave. Box 1239. Brooklyn N.Y. 11203.
Martha Entenmann Tinnitus Research Center, Inc.
Institution: Department Otolaryngology, SUNY/Downstate.
Send correspondence to:
Abraham Shulman
Department Otolaryngology, SUNY/Downstate. 450 Clarkson Ave. Box 1239. Brooklyn N.Y. 11203. Tel: 718 773 8888
E-mail: metrc@inch.com
Paper submitted to the ITJ-SGP (Publishing Management System) by invitation.
Citation: Shulman A, Goldstein B. Electrophysiology quantitative electroencephalography/low resolution brain electromagnetic tomography functional brain imaging (QEEG LORETA): Case report: Subjective idiopathic tinnitus - predominantly central type severe disabling tinnitus. Int Tinnitus J. 2014;19(1):10-27
Abstract
The clinical significance of QEEG LORETA data analysis performed sequentially within 6 months is presented in a case report of a predominantly central type severe disabling subjective idiopathic tinnitus (SIT) before and following treatment. The QEEG LORETA data is reported as Z-scores of z = ± 2.54, p < 0.013. The focus is on demonstration of patterns of brain wave oscillations reflecting multiple brain functions in multiple ROIs in the presence of the tinnitus signal (SIT). The patterns of brain activity both high, middle and low frequencies are hypothesized to reflect connectivities within and between multiple neuronal networks in brain. The Loreta source localization non auditory ROI Images at the maximal abnormality in the very narrow band frequency spectra (24.21 Hz), showed the mathematically most probable underlying sources of the scalp recorded data to be greatest in the mid-cingulate, bilateral precuneus, cingulate and the bilateral caudate nucleus. Clinical correlation of the data with the history and course of the SIT is considered an objective demonstration of the affect, behavioral, and emotional component of the SIT. The correlation of the caudate activity, SIT as the traumatic event with the clinical course of PTSD, and the clinical diagnosis of PTSD is discussed. The clinical translation for patient care is highlighted in a SIT patient with multiple comorbidities by translation of QEEG/LORETA electrophysiologic data, as an adjunct to: provide an objectivity of patterns of brain wave activity in multiple regions of interest (ROIs) reflecting multiple brain functions, in response to and in the presence of the tinnitus signal, recorded from the scalp and analyzed with the metrics of absolute power, relative power, asymmetry, and coherence, for the subjective tinnitus complaint (SIT); 2) provide an increase in the accuracy of the tinnitus diagnosis; 3) assess/monitor treatment efficacy; 4) provide a rationale for selection of a combined tinnitus targeted therapy of behavioral, pharmacologic, sound therapy modalities of treatment attempting tinnitus relief; 5) provide insight into the medical significance of the SIT; 6) attempt discriminant function analysis for identification of a particular diagnostic clinical category of CNS neuropsychiatric disease; and 7) attempt to translate what is known of the neuroscience of sensation, brain function, QEEG/LORETA source localization, for the etiology and prognosis of the individual SIT patient.
Introduction
Electrophysiology quantitative electroencephalography/ low resolution brain electromagnetic tomography functional brain imaging (QEEG LORETA) is recommended to be included into the medical audiologic tinnitus patient protocol (MATPP) for the evaluation and treatment of a predominantly central type severe disabling subjective idiopathic tinnitus (SIT).
The QEEG was introduced by Weller and Brill in the 1990s for diagnosis and treatment of tinnitus patients [1].
Clinical research of the applicability of the QEEG for SIT was performed from 2000-2002 [2]. A descriptive analysis-interpretation of quantitative electroencephalography (QEEG) data for the metric of power in patients with tinnitus of the severe disabling type (N = 61) was reported in 2006 [3].
Since 2006 the QEEG has been introduced into the medical audiologic tinnitus patient protocol (MATPP) for the evaluation and treatment of a predominantly central type severe disabling subjective idiopathic tinnitus (SIT). The electrophysiologic data, correlated with the clinical neurotolgic history and results of the physical examination and clinical course of the SIT, has been provided to the tinnitus professional and SIT patient as an adjunct to increase the diagnostic accuracy for the SIT, attempts to establish its medical significance, translation for selection of treatment modalities attempting tinnitus relief, an objective monitoring function of treatment efficacy, and discriminant function analysis for a identification of a particular diagnostic clinical category of CNS neuropsychiatric disease.
Source localization with LORETA of the maximal abnormality in the very narrow band frequency spectra has identified since 2012 the mathematically most probable underlying sources of the scalp recorded data. The identification of source localization regions of interest images (ROIs) with QEEG/LORETA, has been validated by correlation with nuclear medicine brain imaging single photon emission computed tomography (SPECT) and positron emission tomography (PET) [4-7].
In 2014 QEEG data was presented in a SIT patient with a cochlear implant (CI) soft failure before and after treatment attempting tinnitus relief both with conservative pharmacologic medications and following surgical removal of the initial cochlear implant and reimplantation with a new CI. The utilization and application of the QEEG data provided objective evidence of altered brain wave activity which correlated with the subjective complaint of the SIT patient, i.e. increase of tinnitus intensity with the initial CI and tinnitus relief with a second CI following removal of the initial CI.
It was suggested that a clinical application of the QEEG be extended to all SIT patients 1) to provide objective data to map brain wave activity reflective of multiple brain function activity in the presence of the tinnitus signal and 2) provide an objective method to identify treatment efficacy in terms of “normal”, “abnormal” brain function brain wave activity [8].
In this case report of a patient with SIT and multiple comorbidities, the QEEG/LORETA electrophysiologic data is presented to demonstrate its value as an adjunct to: 1) provide an objectivity of patterns of brain wave activity in multiple regions of interest (ROIs) reflecting multiple brain functions, in response to and in the presence of the tinnitus signal, recorded from the scalp and analyzed with the metrics of absolute power, relative power, asymmetry, and coherence, for the subjective tinnitus complaint (SIT); 2) provide an increase in the accuracy of the tinnitus diagnosis; 3) assess/monitor treatment efficacy; 4) provide a rationale for selection of a combined tinnitus targeted therapy of behavioral, pharmacologic, sound therapy modalities of treatment attempting tinnitus relief; 5) provide insight into the medical significance of the SIT; 6) attempt discriminant function analysis for identification of a particular diagnostic clinical category of CNS neuropsychiatric disease; and 7) attempts to translate what is known of the neuroscience of sensation, brain function, QEEG/LORETA source localization, for the etiology and prognosis of the individual SIT patient.
QEEG LORETA Analysis:
A. Date of Test 12/2014
B. Date of test end 05/2015
Both neurometric QEEG evaluations included a twenty minute recording of eyes closed, resting EEG from which two minutes of artifact-free data were selected for quantitative analysis. All features extracted from the artifact-free EEG were z-transformed relative to age expected normal values and expressed in units of probability (where ± 1.96 is at the p < 0.05 level of significant deviation from normal for their age). This report is based on statistical analyses of the data obtained and is intended to be used as an adjunct to the clinical evaluation of the patient.
A. General Notes/Cautions
- Clinical history: The clinical history is of a 41 y/o/w/m with the chief complaint of tinnitus predominantly ear rt and occasional lt since 09 2014. Significant are the associated complaints of anxiety, increased sensitivity to sound (i.e. hypercusis) since mid 9/2014, ear blockage rt > lt with onset tinnitus, increase tinnitus intensity with pressure application to forehead, face, and clenching of the jaws, stress, weight loss-diet “30” lbs., interference speech expression and memory recent, accompaniment of the tinnitus onset, cervical headache, cervical nuchal discomfort and “tightness”. Recent complaint of imbalance reported 06/2015.
The patient reported a history of head injury sustained in January 2014, with unremarkable MRI findings upon imaging. Patient also reported depression prior to tinnitus onset of approximately 4-6 months duration.
Comorbidities included: preexistent depression, noise exposure, cervical disc, chronic rhinosinusitis, presumed intracranial hypertension, idiosyncratic reactions to anxiolytic/antidepressant/sedative drugs/Eust. tube dysfunction and Secondary endolymphatic hydrops rt - fluctuant. Questionable is PTSD since 06/2015.
B. QEEG/LORETA Test 12/2014
This patient was receiving medication at the time of this evaluation (Xanax). Medications may normalize an abnormal QEEG profile; they may also produce abnormal QEEG features in a normal record, therefore caution should be used in interpretation of this evaluation.
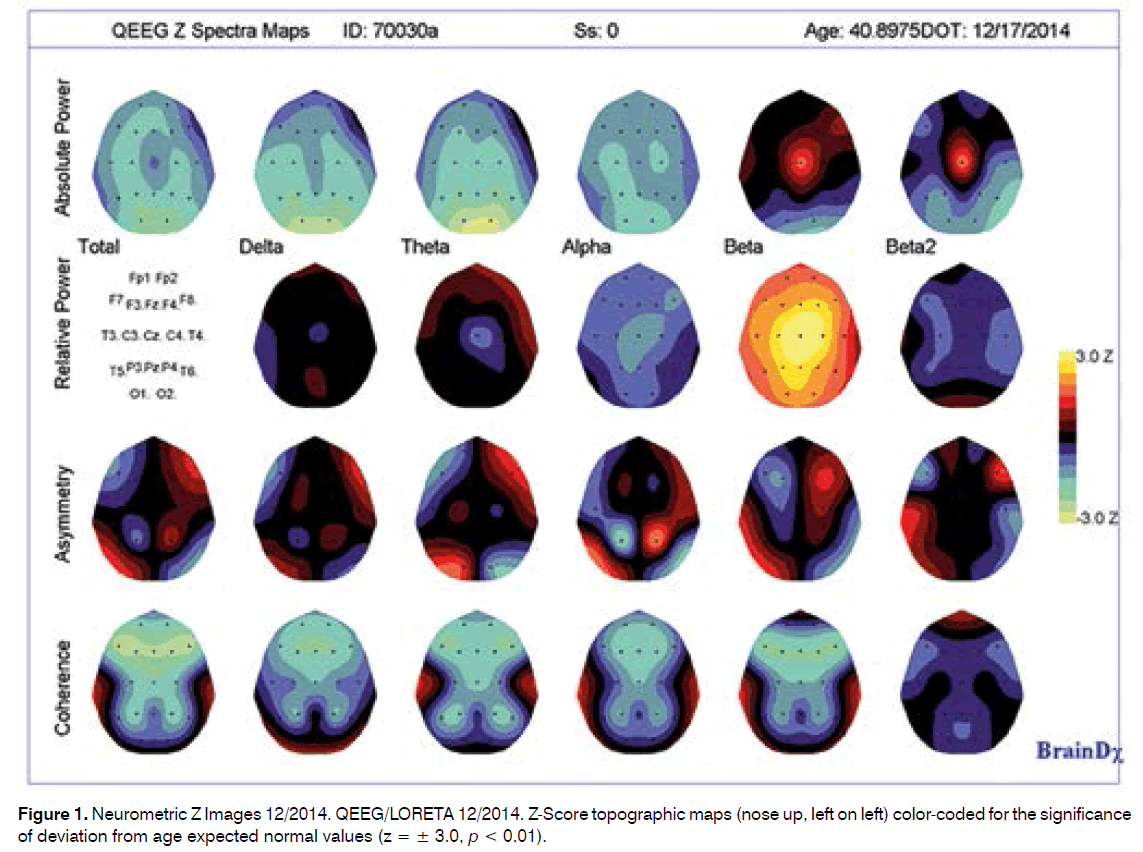
Summary of EEG Findings:
This patient’s neurometric QEEG analysis of the convention (standard) frequencies (delta, theta, alpha, beta and beta 2) was found to be largely within normal limits for his age, although there were some features of note, including:
A tendency toward low power in all frequency bands, only reaching significance in posterior regions in slow waves (delta and theta frequency bands).
A tendency toward excess of relative power (%) in the beta frequency band, only reaching significance in the right central and midline regions.
Significant disturbances in power relationships between and within hemispheres, including:
Within both hemispheres between frontal and occipital regions in slow frequencies (Frnt > Occip), between frontal and midline regions and between midline regions in the beta frequency band (central midline having greatest power).
Within the right hemisphere between anterior and posterior regions, in slow frequency bands and in all cases with more anterior regions having greater power than more posterior regions.
Significant disturbances in coherence and cortical connectivity (coherence relationships between regions), including especially:
Hypocoherences between frontal and more posterior regions and between frontal and central midline regions in the beta band and between frontal regions in the theta band.
Maximal abnormality in the narrow band frequencies was found in the beta band in cortical and subcortical sources.
Details of EEG Findings
This patient’s neurometric QEEG analysis of the broad band frequencies (delta, theta, alpha, beta and beta2) was considered to be largely within normal limits for his/her age. However, there were some features of note, including: [1] A tendency toward diffuse low power in all frequency bands, only reaching significance in P3, P4, O1 and O2 posterior regions in delta and theta frequency bands; [2] A tendency toward diffuse excess of relative power (%) in the beta frequency band, only reaching significance in the C4, Cz and Fz region. A significant peak was found in the very narrow band frequency spectra at 24.21 Hz, maximal at the vertex (central midline regions).
Significant disturbances in power relationships were found, including:
a. within both hemispheres between frontal and occipital regions (Fnt > Occip, delta, theta, beta) and between parietal and occipital regions (p > O, delta and theta);
b. within the right hemisphere between P4 > O2, F8 > T4, F8 > T6, C4 > Cz (delta) and T4 > T6 (theta);
c. within both hemispheres and midline regions between FP1 < Cz, F7 < Cz, Fz < Cz, Pz > Cz, P4 > O2, C3 < Cz, C4 < Cz in the beta and beta 2 bands.
Significant disturbances in coherence and cortical connectivity (coherence relationships between regions), including especially:
Hypocoherences between frontal and more posterior regions and between frontal and central midline regions in the beta band and between frontal regions in the theta band;
Hypocoherences in total power between frontal regions and contralateral short distance regions, and between right anterior temporal regions and short and long distance ipsilateral and midline regions;
Hypercoherences in delta, theta and alpha bands between left dorsolateral prefrontal region and ipsilateral short distances.
Visual inspection of the EEG record revealed beta bursting throughout the record at the vertex region.
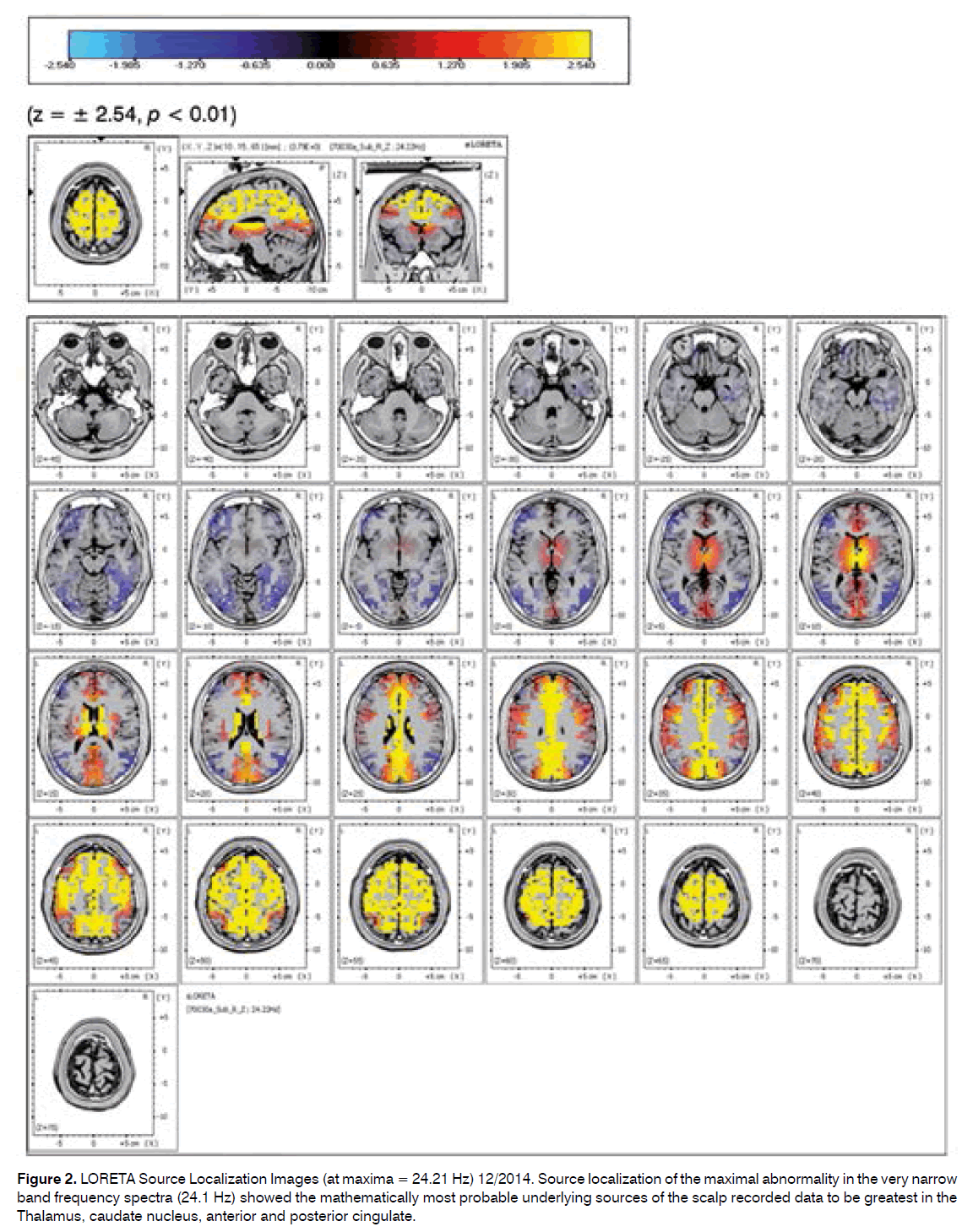
Source localization of the maximal abnormality in the very narrow band frequency spectra (24.1 Hz) showed the mathematically most probable underlying sources of the scalp recorded data to be greatest in the Thalamus, caudate nucleus, anterior and posterior cingulate.
C. Date of test end 5/2015
General Notes/Cautions: This patient was receiving medication at the time of this evaluation (Klonopin). Medications may normalize an abnormal QEEG profile; they may also produce abnormal QEEG features in a normal record, therefore caution should be used in interpretation of this evaluation.
The patient reported a history of head injury sustained in January 2014, with unremarkable MRI findings upon imaging. Patient also reports history of tinnitus since September 2014 and current depression, cervical disc.
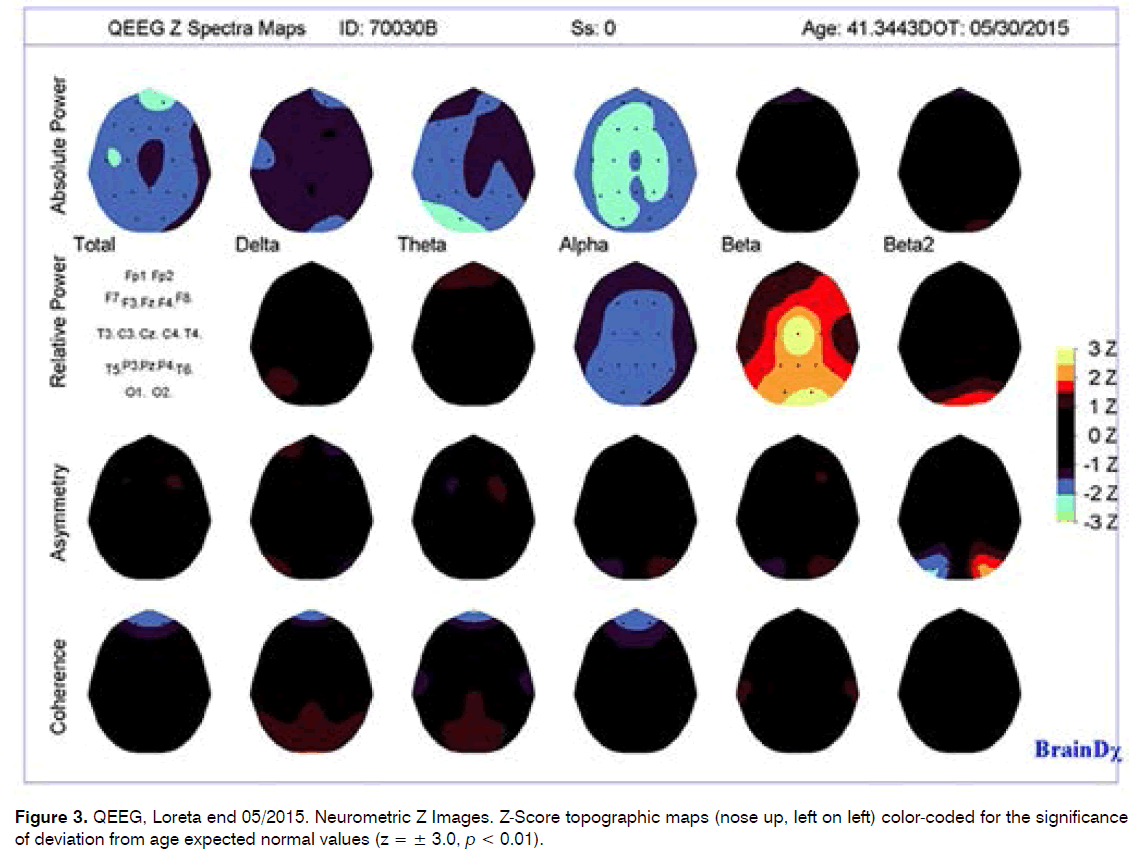
- Summary of Quantitative EEG Findings
This patient’s neurometric QEEG analysis of the convention (standard) frequencies (delta, theta, alpha, beta and beta2) was found to be largely within normal limits for his age, although there were some features of note, including:
A tendency toward low power in all frequency bands, reaching significance in alpha band especially in the central, parietal and occipital regions and in the theta band in the occipital, temporal and fronto-occipital regions.
A tendency toward excess of relative power (%) in the beta frequency band, only reaching significance in the right parietal and bilateral occipital regions.
Significant disturbances in power relationships between midline central and frontal, temporal and central regions, with more power in the central midline region.
Coherence and cortical connectivity (coherence relationships between regions), was considered to be within normal limits.
Maximal abnormality in the narrow band frequencies was found in the beta band in cortical and subcortical sources.
- Details of EEG Findings
This patient’s neurometric QEEG analysis of the broad band frequencies (delta, theta, alpha, beta and beta 2) was considered to be largely within normal limits for his age. However, there were some features of note, including: [1] A tendency toward diffuse low power in all frequency bands, reaching significance in F3, Fz, C3, C4, P3, P4, O1, O2, T5 regions in alpha, and in the bipolar temporal, central-parietal and fronto-occipital regions in alpha and in occipital regions in the theta frequency bands; [2] A tendency toward diffuse excess of relative power (%) in the beta frequency band, only reaching significance in the P4, O1 and O2 regions and a tendency toward a diffuse deficit of alpha; [3] Mean frequency showed a tendency toward decrease in the theta band. A significant maxima peak was found in the very narrow band frequency spectra at 23.8Hz, maximal at the vertex (central midline region).
Significant disturbances in power relationships were found between the central midline regions and frontal regions (F < Cz) and between central and parietal midline regions (Cz > Pz) in the beta bands. Coherence relationships were found to be largely within normal limits for his age. There were a few inter-regional coherence relationships which were of note as seen in the images below.
Visual inspection of the EEG record revealed occasional small sharp waves (especially on left hemisphere).
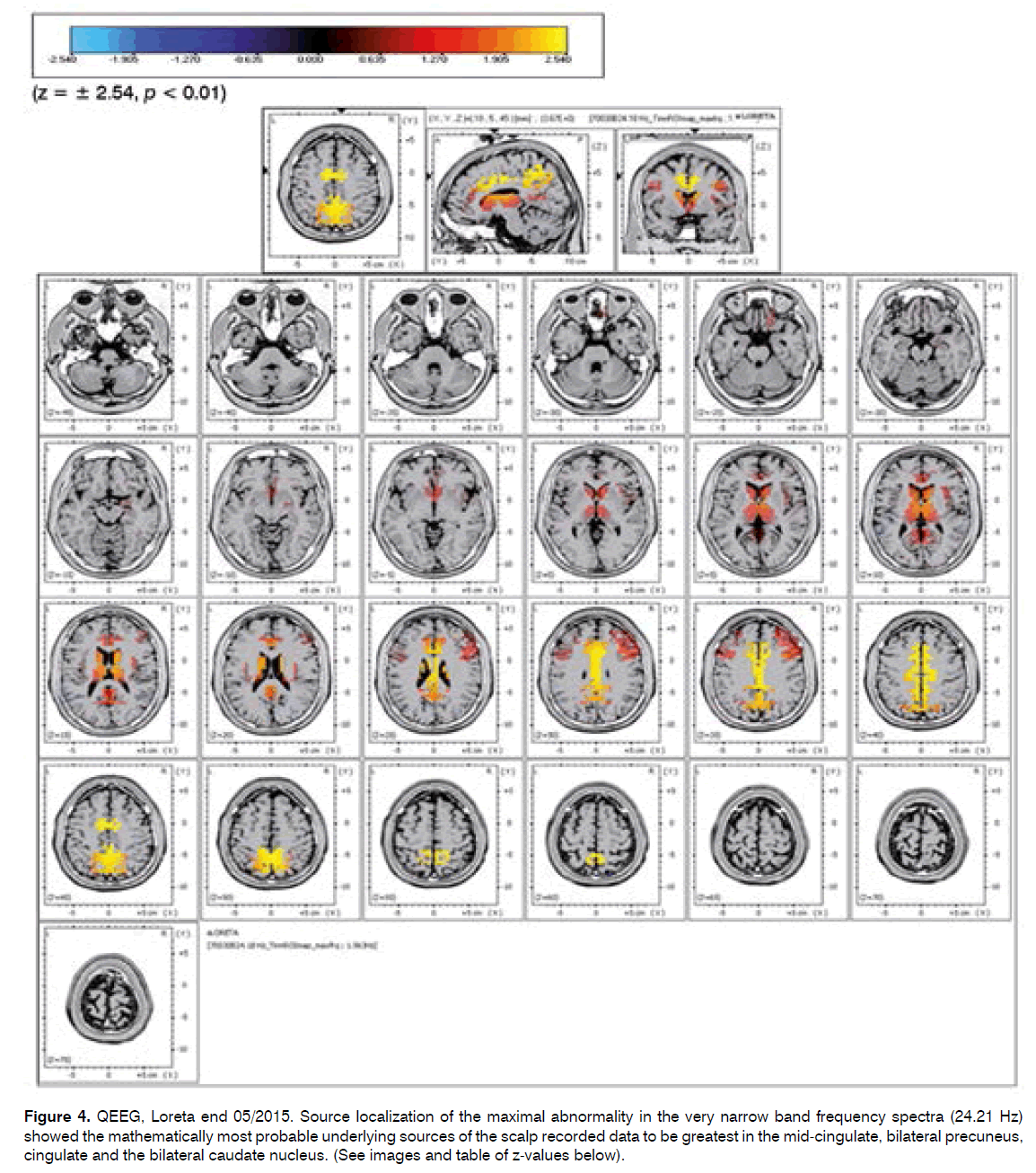
Source localization of the maximal abnormality in the very narrow band frequency spectra (24.21 Hz) showed the mathematically most probable underlying sources of the scalp recorded data to be greatest in the mid-cingulate, bilateral precuneus, cingulate and the bilateral caudate nucleus. (See images and table of z-values below).
Comparisons to the neurometric database to evaluate the likelihood that a patient’s neurometric profile statistically resembles that seen in patient populations with specific clinical conditions should be made in the drug-free condition and not in the presence of head injury. Thus, caution must be used in comparing this patient to those in the database. However, it is noted that this patients profile did not result in a classification as belonging to the population with major affective disorder.
Comparison with Prior Examination 12/2014
While both evaluations showed a tendency toward low power in all frequency bands, the prior exam found this to be most noted in delta and theta, while the current evaluation found this to be maximal in the alpha and theta bands, suggesting a shift in the frequency spectrum. The tendency toward excess of relative power (%) in the beta frequency band seen in the prior evaluation was also seen in this evaluation although more posterior. With respect to power relationships between and within hemispheres, both evaluations found this to be maximal in the beta frequency band and involving midline central regions. The significant disturbances previously reported in coherence and cortical connectivity (coherence relationships between regions), were found to be largely normalized in this evaluation.
Narrow band abnormalities and maximal peak in the LORETA spectra were found in both evaluations to be in the high beta frequency band with most probable underlying sources of the scalp recorded data to be greatest in similar regions with the current evaluation showing more involvement of the precuneus and less involvement of the Thalamus compared with the prior exam.
Case Report
History/Chief complaint
The patient was seen in initial consultation mid 12/2014 for the chief complaint of an increase in tinnitus intensity since 2/2014 which was reported to have the following characteristics:
• Quality - described as a ”constant high pitched noise in quiet ear right”, overall in ears lt > rt, and “greater variety of sound qualities of additional frequencies” described as “more than one tone”, “loud ringing and high pitched spikes”.
• Location - reported predominantly ear lt > ear rt,
• Intensity - fluctuant.
• The duration of the tinnitus reported to be constant ear lt > ear rt
• Pulsation - absent
The annoyance of the tinnitus was reported initially 09/2014 to be severe and included interference in sleep, concentration, communication, task performance, and social activities and increase in intensity ion “loud noise environments”.
Associated complaints included increased sensitivity to sound (i.e. hypercusis) since 01/2014, hearing loss “particularly high frequencies” - gradual in onset since 11/2012, facial pain, ear blockage lt with no correlation with tinnitus intensity -intermittent since 01/2013, otalgia intermittent ear lt since 07/2013, head pressure, “dizziness” intermittent since 05/2014, and occasional headache.
No complaint loss of consciousness.
Clinical course: 09/2014-12/2014
Tinnitus initial onset, described as when patient was awake and “hours” following exposure to the noise of a race car “greater than 100 dB”. The tinnitus severity was “immediate”, with “anxiety and panic”, associated ear blockage bilateral rt > lt, and hypercusis.
The clinical course of the tinnitus since 09/2014 has been marked by a gradual increase in intensity, severity, and annoyance since 9/14 with intervals of significant improvement in tinnitus intensity. Intervals of tinnitus relief were accompanied by increased complaints of ear blockage bilateral rt > lt, neck pressure, head pressure, occasional and transient paresthesia hand and/or fingers rt and increasing depression. Expressions of futility were expressed from the initial date of neurotolgic consultation and psychiatric consultation was requested for attempt control antecedent, prior to 09/2-1014, and increased reported overall depression associated with the SIT.
Significant clinical history has been the identification of an antecedent depression prior to 09/2-1014, i.e. since 07/2014 - family/work stress related-history. Increased anxiety/depression was reported during the clinical course of the tinnitus.
Tinnitus intensity increase and ear blockage rt decrease reported with supine position. Paresthesia described as “tingling” right hand and fingers- summer 2014. Speech and memory interference was reported to accompany the tinnitus onset.
The initial quality of the tinnitus was the “tea kettle”, followed by the “steam “a month later. The increased tinnitus intensity with pressure on the forehead and jaw clenching was noted immediately, i.e. somatosensory component. Increased intensity tinnitus reported “in quiet” “at night” and accompanied by increased anxiety.
An overall weight loss of 30 lbs, diet related, was 15 lbs prior to the tinnitus onset and additional 10-15 lbs. since the onset of the tinnitus.
No reported tinnitus pulsation. Imbalance complaint(s) reported intermittently since 6/2015 and described as head pressure and “cheap drink” sensation.
Lymphadenopathy in the nuchal region reported by the patient 01/12/15, was evaluated by infectious disease 01/15/15. The node was reported to be singular, not associated with other regional lymphadenopathy, blood test abnormality. The result of the lymph node biopsy was clinically considered to be negative. Report has been requested. An ulcer of the lip was identified, biopsied and reported “negative for herpes” by infectious disease 01/15/15. Clinically the clinical course of the tinnitus as described was not affected.
Significant multiple comorbidities and risk factors elicited from the clinical history were identified, respected and evaluated for potential contribution to the clinical course of the tinnitus as described -alone and/or in combination:
• Antecedent to 12/2014-
• Head trauma - ski accident - 01/2014
• Infectious disease - Lyme disease 08/2014 - reported negative.
• Noise exposure - sport racing car - 9/2014
• Depression/Stress-ongoing prior to 09/2014
• Since 09/2014:
• Anxiolytic/Antidepressant/Sedative medication - reduction dosage Prozac/Ambien “mid 9/2014.
• Idiopathic intracranial hypertension presumptive diagnosis - Position related alteration in tinnitus intensity and degree of ear blockage rt/lt since 03/2014.
• Fluctuation in aeration of the middle ears rt > lt - yrs duration, intermittent.
• Secondary endolymphatic hydrops rt -fluctuant.
• Chronic rhinosinusitis- yrs duration - controlled.
The complexities of the SIT in this patient were reflected in the clinical course of the SIT as described from 09/2014 - start 6/2015. Multiple comorbidities were identified in the clinical history. And during the clinical course of the SIT. The results of the tinnitus evaluation and cochleovestibular test battery, correlated with the clinical history and neurotolgic physical examination established the clinical tinnitus diagnosis of a predominantly central type severe disabling tinnitus with cochlear, middle ear and somatosensory components bilateral and to have been subclinical ear rt and lt prior to date of onset of the tinnitus 09/2014.
Since mid 5/2015 the associated complaint of depression/anxiety has increased in severity becoming the priority complaint with replacement of the original SIT, particularly at intervals of reported tinnitus relief.
Visual analog scales of intensity and depression, the Tinnitus handicap inventory, Beck’s depression index- all - were consistent with SIT. - 12/2015 4 and 06/2015.
Clinical impressions 12/2014
Impressions:
1. Conventional audiometry 250-8000 Hz:
• Hearing loss -Ear rt: normal peripheral hearing sensitivity 250-8000 Hz
• Ear Lt: normal peripheral hearing sensitivity 250-4000 Hz to mild hearing loss at 4, 8 kHz.
• Speech recognition thresholds corroborated these results. Word recognition scores excellent bilateral.
2. Ultrahigh frequency audiometry 10-20 kHz, ear rt SPL thresholds at 12.5, 14 kHz reduced with no response at 16 kHz Hz;
ear lt SPL thresholds reduced at 16 kHz, greater than expected for the age level of the patient to the limits of the audiometer 120 dB SPL.
3. Comparison 10/6/14-12/24/14 conventional audiometric examinations 250-8000 kHz-audiometric pattern essentially unchanged. Asymmetry 4kHz lt.
4. Comparison 10/6/14-12/24/14 conventional audiometric examinations 250-8000 kHz-audiometric pattern essentially unchanged. Asymmetry 4kHz lt.
5. Tinnitus a predominantly central type severe disabling tinnitus with cochlear, middle ear and somatosensory components bilateral and to have been subclinical ear rt and lt prior to 09/2014.
6. Hypercusis - history.
7. Secondary endolymphatic hydrops rt - presumptive.
8. Eust. Tube obstruction bilateral-presumptive ear rt > lt; controlled at this time bilateral.
9. Chr. rhinosinusitis.
10. Anxiety Syndrome/depression - History.
11. Head trauma; Cerebral Concussion - history 01/2014.
12. Idiopathic Intracranial hypertension- presumptive
13. RO Secondary endolymphatic hydrops rt > lt; CNS Cognitive disorder; TMJ Syndrome; Ski accident 01/2014 - Post concussion Syndrome.
Clinical Course of the tinnitus 12/2014 - 06/2015
1. Tinnitus relief:
• Overall -fluctuant; intermittent, with gradual improvement, maintained 01/13-1/2015 I.e. predominantly improvement in mood/sleep, mild reduction in tinnitus intensity and annoyance and ear blockage ear rt > ear lt- considered to be significant to the patient.
• Improvement a reflection of completion of recommendations to establish an accurate diagnosis for the tinnitus as described 12/12/2014 and to attempt tinnitus relief:
• Stage 1: treatment recommendations 12/12/2014 - target complaint(s) tinnitus, ear blockage rt, depression; sleep-combination sound attenuation; natural clinical course following recovery cerebral concussion; attempt improvement Eust. Tube function; general rules; continuation psychiatry FUVS prior to onset tinnitus mid 09/2014.
• Stage 2: 01/09/2015 0 - Continuation initial recommendations/Target secondary endolymphatic hydrops presumed rt-reduction ear blockage rt/addition trial diuretic treatment.
• Fluctuation in improvement in the ear blockage ear rt ranged from 30-40% to 10-20%. Episode of noise exposure resulted in an increase in the ear blockage ear rt > ear and elimination of the improvement, 30-40% reported 01/09/2015. Restoration of ear blockage reduction ear rt to 10-20% reported 01/13/2014 to 01/19/15. Continuation diuretic with replacement Dyazide with Diamox 250 mg alternate days.
• Stage 3: Psychiatry consultation - secondary opinion evaluation treatment depression antecedent to and following tinnitus onset mid 09/2014.
• Instrumentation for tinnitus relief to be determined by clinical curse of the patient.
2. Tinnitus quality/duration - ear rt-constant, quality “high pitch hiss” 12/2014; ear rt.
3. Sound attenuation therapy- tinnitus relief, TII 0-2; residual inhibition increased from approximately “a minute” to “several minutes”.
4. Tinnitus intensity fluctuation - frequent - Annoyance reduced in AM - TII 2-3 during the day; increase intensity and annoyance in Pm- TII. 3-4; no pulsation.
5. Hearing loss - denied.
6. Hypercusis - significant reduction - bilateral - with sound attenuation.
7. Ear blockage ear rt > ear lt fluctuant; reduced rt 10-20% and maintained since 01/13/2015.
8. No compliant(s) - head pressure, pulsation;
9. Trial instrumentation: Neuromonix - rebound effect - incomplete follow-up; tinnitus masker - incomplete; Tinnitus retraining therapy - incomplete.
10. Trial Intratympanic therapy steroid ear rt - early 5/2015 x2- transient dramatic reduction with second trial - incomplete.
11. Epidural cervical steroid injection x2 - 5-6/2015 - improvement complaint neck pressure; elimination paresthesias fingers rt.
9. Headache - nuchal and frontal lt - occasional, reduced frequency occurrence 01/17/15.
10. Head pressure/vertigo absent.
11. Sleep interference reported improvement “better” 5-7 hrs duration reported 01/19/15; predominantly interrupted increasing intervals 3-6/2015.
12. Mood/sleep - improvement associated with start Klonopin. 25 mg hs; replacement for Xanax- since 01/11/15.
13. Klonopin. 25 mg HS. - “improvement mood”. Patient supplements recommended dosage with Klonopin. 25 mg in mid morning following when awake.
14. Psychiatry - 03/2015-05/2015 attempt mood control ongoing trial Wellbutrin; Celexa; 06/2015 Lexapro/ Gabapentin.
Discussion
The points of discussion include the following: A. QEEG/LORETA DATA: 12/2014; end 05/2015 From a clinical perspective, what are we “seeing” with the QEEG/LORETA in this SIT patient?
Answers:
a. Electrophysiolgic data - a spectral analysis of the raw EEG data for frequencies of brain wave activities analyzed for the metrics of absolute power, relative power, asymmetry and coherence.
b. Source localization- the maximal abnormality in the very narrow band frequency spectra which shows the mathematically most probable underlying sources of the scalp recorded data.
c. Multiple brain functions in multiple regions of interest (ROIs) in brain in the presence of or response to internal and/or external sensory stimulation.
d. Comparison of frequencies of brain wave activities at two sequential test dates of a 6 month interval of time.
e. Electrophysiologic and source localization data of comorbidities coincident with the chief complaint of the patient.
A spectral analysis of the raw electroencephalographic data, i.e. QEEG, for the metrics of absolute power, relative power, asymmetry, coherence - both hyper and hypo coherences and cortical connectivity, when compared to a normative data base, i.e. Neurometrics, identifies and provides a mathematical, and objective standard of deviation from the normal. The clinical neurotolgicaudiologic history, and physical examination identified multiple comorbidities, both primary and secondary, highlighted not only by “tinnitus” and the head trauma, but components of the SIT. The LORETA analysis provided a source localization for the scalp surface recorded electrical potential. Together, the QEEG LORETA analysis provides to the tinnitus professional and patient a mathematical and visual display of the electrical activity in brain for normal and abnormal aberrant brain function.
B. Comparisons QEEG/LORETA 12/2014; end 05/2015
Comparison end 05/2015 with 12/2014 identified the narrow band and abnormalities and maximal peak in the LORETA spectra were found in both evaluations to be in the high beta frequency band with most probable underlying sources of the scalp recorded data to be greatest in similar regions with the evaluation end 5/2015 showing more involvement of the precuneus and less involvement of the Thalamus compared with the prior exam and persistence of statistical significance of the Z-score of the caudate rt > lt Clinically hypothesized to reflect a consolidation a memory and emotional response to the trauma of the SIT (Figure 4).
While both evaluations showed a tendency toward low power in all frequency bands, the prior exam found this to be most noted in delta and theta, while the current evaluation found this to be maximal in the alpha and theta bands, suggesting a shift in the frequency spectrum. The tendency toward excess of relative power (%) in the beta frequency band seen in the prior evaluation was also seen in this evaluation although more posterior. With respect to power relationships between and within hemispheres, both evaluations found this to be maximal in the beta frequency band and involving midline central regions. The significant disturbances previously reported in coherence and cortical connectivity (coherence relationships between regions), were found to be largely normalized in this evaluation.
The QEEG/LORETA data provides a map of the fluctuation brain wave activity in the clinical course of the SIT patient. Comparison end 05/2015 with prior Examination 12/2014 revealed:
1) The low power in all frequency bands was identified in both evaluations, initially in the delta and theta, while the end 5/2015 evaluation found this to be maximal in the alpha and theta bands, suggesting a shift in the frequency spectrum. Good prognosis for HT; need to separate for SIT and etiology Figure 1 and 3).
Figure 2. LORETA Source Localization Images (at maxima = 24.21 Hz) 12/2014. Source localization of the maximal abnormality in the very narrow band frequency spectra (24.1 Hz) showed the mathematically most probable underlying sources of the scalp recorded data to be greatest in the Thalamus, caudate nucleus, anterior and posterior cingulate.
2) The tendency toward excess of relative power (%) in the beta frequency band seen in the 12/2014 evaluation was also seen in this evaluation although more posterior. HT (Figure 1 and 3).
3) With respect to power relationships between and within hemispheres, both evaluations found this to be maximal in the beta frequency band and involving midline central regions (Figures 1, 2, 3, 4 and 5).
Figure 4. QEEG, Loreta end 05/2015. Source localization of the maximal abnormality in the very narrow band frequency spectra (24.21 Hz) showed the mathematically most probable underlying sources of the scalp recorded data to be greatest in the mid-cingulate, bilateral precuneus, cingulate and the bilateral caudate nucleus. (See images and table of z-values below).
4) The significant disturbances previously reported in coherence and cortical connectivity (coherence relationships between regions), were found to be largely normalized in this evaluation. Clinically this is a demonstration of the monitor function of the QEEG for treatment efficacy (Figures 1, 2, 3, and 4).
The data from two sequential (N = 2) QEEG/ LORETA tests in this case report in a patient with SIT with the cited multiple comorbidities, highlighted by head and neck trauma, cervical disc, depression, tinnitus, provides a demonstration to both patient and tinnitus professional, of the following:
1) multiple patterns of brain wave activities reflective of activation of multiple brain functions in the presence of the tinnitus signal and multiple comorbidities, for the metrics of absolute power, relative power, asymmetry of power and coherence- the connectivity of electrical activity between and within ROIs, hypothesized at neuronal and synaptic levels of activity;
2) objective evidence of CNS brain wave abnormal activity in ROIs in support of the clinical diagnosis of a central type tinnitus;
3) a monitor function of the QEEG/LORETA to identify the natural clinical course of the tinnitus, positive and negative, and response to modalities of treatment attempting tinnitus relief i.e. a reduction in the overall brain wave activity for each of the metrics of the data;
4) a recovery and or efficacy of treatment function of the QEEG/LORETA of the natural and artificial modalities of treatment, eg Klonopin attempting tinnitus relief,
5) support for and continuation of the existing rationale underlying selection of modalities of treatment attempting tinnitus relief, in particular Klonopin for its GABAergic inhibitory action, and consideration of addition of a dopaminergic drug for attempting tinnitus relief;
6) adjunct function clinically considered to support the central component of the tinnitus diagnosis; and depression.
7) discriminant function analysis for specific comorbidities based on the data and its correlation with the clinical history, eg Head and neck trauma, and depression.
The hyper and hypo connectivities in and between the central electrode recording site(s), the caudate nucleus and insula, and the source localization of maximal abnormality in the narrow band frequency spectra (24.21 Hz), mid-cingulate, bilateral precuneus, cingulate and the bilateral caudate nucleus clinically is hypothesized to reflect pathophysiological processes between the cortex and striatum involving the dopamine system.
Significantly, in this patient the hyper and hypo connectivities and the source localization data in both studies are non auditory and not auditory ROIs.
Narrow band abnormalities and maximal peak in the LORETA spectra were found in both evaluations to be in the high beta frequency band with most probable underlying sources of the scalp recorded data to be greatest in similar regions with the current evaluation showing more involvement of the precuneus and less involvement of the Thalamus compared with the prior exam.
The source localization 12/2014 of the maximal abnormality in the very narrow band frequency spectra (24.1 Hz) showed the mathematically most probable underlying sources of the scalp recorded data to be greatest in the Thalamus, caudate nucleus, anterior and posterior cingulate.
The source localization end 05/2015 showed the mathematically most probable underlying sources of the scalp recorded data to be greatest in the bilateral precuneus, cingulate and the bilateral caudate nucleus caudate rt > lt Z 2.39 rt2.21 lt, and less involvement of the Thalamus compared with the prior exam.
The QEEG/LORETA 05/2015 data provides a map of the fluctuation brain wave activity in the clinical course of the SIT patient.
Comparison with Prior Examination 12/2014 revealed:
1) The low power in all frequency bands was identified in both evaluations, initially in the delta and theta, while the end 5/2015 evaluation found this to be maximal in the alpha and theta bands, suggesting a shift in the frequency spectrum. Good prognosis for HT; need to separate for SIT and etiology (Figure 1 and 3).
2) The tendency toward excess of relative power (%) in the beta frequency band seen in the 12/2014 evaluation was also seen in this evaluation although more posterior. HT (Figure 1 and 3).
3) With respect to power relationships between and within hemispheres, both evaluations found this to be maximal in the beta frequency band and involving midline central regions (Figure 1, 3 and 4).
4) The significant disturbances previously reported in coherence and cortical connectivity (coherence relationships between regions), were found to be largely normalized in this evaluation. Clinically this is a demonstration of the monitor function of the QEEG for treatment efficacy.
The significant disturbances previously reported in coherence and cortical connectivity (coherence relationships between regions), were found to be largely normalized in this evaluation with persistence of the ROIs caudate and cingulate.
C. Clinical Course SIT 12/2014 - 6/2015 and Comorbidities
Clinically, the Electrophysiological QEEGLORETA 12/2014, end 5/2015 data in brain of this patient are considered to be reflective of disturbances in the “normal” electrical brain wave activity of brain in who multiple comorbidities both primary and secondary were identified.
Primary comorbidities were highlighted by “tinnitus” and head trauma, but by components of the aberrant auditory sensation, the SIT, i.e. sensory, affect behavior emotion, psychomotor, memory, and chronicity. Secondary comorbidities were highlighted by a preexistent depression, noise exposure, cervical disc, chronic rhinosinusitis, presumed intracranial hypertension, idiosyncratic reactions to anxiolytic/antidepressant/ sedative drugs/Eust. Tube dysfunction and Secondary endolymphatic hydrops rt - fluctuant.
Clinically It is hypothesized, that the QEEG/ORETA tests disturbances reflect a global physical displacement effect on the intracranial fluid and solid brain contents, within and between frontal and posterior occipital, frontal and midline regions, between and within central midline regions, and between temporal and midline regions. Clinically the physical displacement can be explained and accepted as movement of intracranial contents, solid and fluid, within a closed container, the skull. The multimetric QEEG patterns of analysis of the spectra of frequency(ies) of the brain wave activities, delta, theta, alpha, beta, and beta 2 do not provide to the clinician and the patient a specificity for a particular etiology for the tinnitus but a sensitivity for the functional insult imposed upon multiple structures of the brain by the head trauma in the presence of the preexistent comorbidities.
The focus of the initial neurotolgic evaluation 12/2014 was on the sensory component of the SIT for diagnosis and treatment. Simultaneous Initial psychiatric consultation was obtained for the affect behavioral emotional component of the SIT. Fluctuant tinnitus relief was reported, and identified by visual analog scales. Coincident with the tinnitus relief was in mid 5/2015 an increase in the affect component Episodes of increased SIT intensity were reduced with supplemental Klonopin. Significantly, in mid 5/2015, coincident with reports of significant tinnitus relief, an increase in the affect component of depression was identified. The QEEG end 5/2015 originally planned following the QEEG 12/2014 was repeated. Clinically, both QEEG/LORETA tests showed a tendency toward low power in all frequency bands. The significant disturbances previously reported in coherence and cortical connectivity (coherence relationships between regions), were found to be largely normalized in this evaluation. There is a persistence of elevated Z-scores for the caudate ROI source at 24.21 Hz in the QEEG/LORETA end 5/2015.
Clinically the QEEG test 12/2014 was considered to reflect the multiple comorbidities coincident with the SIT. A basis for innovative pharmacologic GABAergic medication, i.e. Klonopin, was recommended and commenced 01/2014 with a trial of Klonopin. 25 mg in mid morning when awake. Initial report was of Improvement in mood; and the SIT to be unchanged. The Klonopin dosage between 01/2014 and 6/2014 was recommended in stages; 1) Stage 1 Klonopin. 25 mg hs; Stage 2) Klonopin. 25 mg based on cycle reported of tinnitus intensity.
The cycle repeated daily included; a) tinnitus intensity reduced and best in AM with arising, b) increase intensity with assuming erect position and activities in AM in early morning, c)gradual increase with plateau early afternoon, i.e. 12-1PM and maintained with minimal fluctuation until return late afternoon - before bedtime; d) gradual increase tinnitus intensity following assuming supine position; and e) increase maximal intensity with awakening from sleep and reduced total hours of interrupted sleep. Accordingly Klonopin dosage recommended was a) Klonopin .125 mg; b) .125 mg 12-1PM; c) .125 mg 5PM; d). 5 mg 10-11PM Hs. - total = 1.87, i.e. .875 mg/24 hrs. When the tinnitus intensity index, (TII), a visual analog scale of 0-7 where 0 is no tinnitus perception and 7 = worst intensity - if 5 or above - supplemental. 25 mg and repeat in 4 hrs if no tinnitus relief, i.e. supplemental total not to exceed. 1.5 mg in 24 hrs.
QEEG/LORETA was recommended end 05/2015 to provide adjunct information to attempt clinically to establish an accuracy for the tinnitus diagnosis, and as a monitor to identify the efficacy of the tinnitus targeted therapy. The tinnitus relief, intermittent since end 4/2015, was reported to fluctuate with range of occasional “0” and average of 2-3 in Am and 3-. Trial of Neuromonix and masking resulted in a “rebound effect, ie tinnitus intensity increase during and following sound therapy. Tinnitus relief was reported during sound attenuation with white noise. However, instrumentation sound therapy trial was deferred by the patent. The initial hypercusis has been reported to be “no longer a complaint”. The somatosensory component was reported to be occasional with residual pressure point on neck/face/ and grinding of teeth. The sleep was interrupted but improved. A trial of Intratympanic steroid for tinnitus relief ear rt was attempted twice in 05/2014. Initially the tinnitus intensity increased within 24 hours and was reduced to a TII = 0, hours duration with average of 2 in the following 24 hours of days duration and return to average TII 2-3 in the Am with arising from sleep and average of 3-4 during the day. The patient reported the tinnitus, i.e. the sensory component of the tinnitus not to be a problem - But - the affect mood, emotional behavioral component to be the issue since end 05/2014 - early 06/2014.
A significant gradual increase in the affect behavior emotional component of the tinnitus became clinically manifest from mid 5/2015, which has become the predominant component of the SIT. A second psychiatric opinion was recommended. The clinical impression that the clinical course of the SIT be viewed from the paradigm of a medical trauma was proposed by a second psychiatrist. The onset of tinnitus, is perceived as a severe stressor over which the patient has no control) and a lingering Post-Traumatic Stress Disorder. As such, the patient is sensitized by the initial onset of tinnitus symptoms, experiencing intense anxiety as this new diagnosis represents a constant irritant, a loss of his ‘perfect’ self, and a regular reminder of that imperfection. This is a severe and unrelenting narcissistic injury for the patient who is an attractive, masculine husband, father, and entrepreneur with no prior medical or psychiatric history. He becomes hyper vigilant (i.e. always on guard) for the next irritant which may exacerbate his tinnitus, be it a glass of wine, a loud party, the hum of a computer or even the putative hum of the lightbulbs in his home (this is all verified by his wife). His level of hypervigilance is more than merely psychological; his sympathetic nervous system is overactive. An overactive sympathetic nervous system has a positive feedback effect on tinnitus intensity. He is at risk for developing avoidant behavior to limit exposure to perceived sounds which might exacerbate tinnitus and the distress it causes.” Initial reports of depression control are positive for the first time since 12/2014 with a combined approach for control depression with psychiatry, pharmacology, and psychology [9]
The significance of the caudate scores in this patient, specifically for a correlation with PTSD, to be determined by the clinical course of the SIT patient.
Summary QEEG/LORETA 12/2014; end 05/2015:
The initial EEG/LORETA 12/2014 data identified the complexities of the patterns of brain wave activities accompanying the multiple brain functions in the presence of the aberrant auditory sensation, i.e. SIT, and multiple comorbidities. The central component of the tinnitus diagnosis was objectively identified with the QEEG/Loreta and support for a pharmacologic trial, of an antiepileptic, i.e. Klonopin. The severity of the head trauma, and significance of the antecedent depression, both not visible with structural MRI of brain was established.
Clinically to be considered is whether the residual alterations in the QEEG/LORETA end 5/2014 are signs of chronicity for the SIT and cognitive complaint complication of the head injury 01/2014.
The Qeeg/LORETA end 5/2015 reflects multiple brain function responses to multiple comorbidities highlighted by the SIT.
D. Caudate Nucleus:
The Z score for the caudate rt > lt ROI may clinically reflect PTSD in this patient in which the initial trauma was the tinnitus. The Z-scores for the caudate in the end 05/2015 QEEG/LORETA were both statistically significant. i.e. rt 2.39 and lt 2.21, rt > lt.
The caudate nucleus is a structure that has been implicated in the neural circuitry of psychological responses to trauma. A study design was aimed to quantify the volume of the caudate in persons exposed to trauma, after adjustment for the covariates (age, sex, intracranial volume, years since trauma, and number of trauma episodes). A significant difference was reported in the right caudate nucleus volume between subjects with PTSD compared with those without PTSD. Volume of the left caudate nucleus was not significantly different between the PTSD and no PTSD groups. The right caudate volume in the PTSD group was 9% greater compared with the no PTSD group. There is a larger right hemisphere volume of the caudate within those exposed to trauma with active PTSD compared with those without PTSD, superimposed upon a baseline caudate asymmetry [10].
In general, converging evidence was reviewed from multiple domains including anatomical studies of corticostriatal circuitry, neuroimaging studies of healthy volunteers, patient studies of performance deficits on a variety of cognitive tests, and animal studies of behavioral control. A modular conception of the striatum, consistent with hierarchical models of corticostriatal function through which adaptive behavior towards significant goals can be identified i.e. (motivation; ventral striatum), planned (cognition; caudate) and implemented (sensorimotor coordination; putamen) effectively. It was concluded that the caudate nucleus contributes to behaviour through two processes: 1) the excitation of correct action schemas and 2) the selection of appropriate sub-goals based on an evaluation of action-outcomes. Both processes are considered to be fundamental to successful goal-directed action. The putamen, in contrast, appears to subserve cognitive functions more limited to stimulus-response, or habit, learning [11].
Clear evidence has been demonstrated of an association between a smaller amygdala volume and PTSD. Amygdala and hippocampal volumes were computed from automated segmentation of high-resolution structural 3-T magnetic resonance imaging in a case-controlled design with structural magnetic resonance imaging and clinical diagnostic assessments [12].
The role of the caudate has also been hypothesized based on a model - caudate/insula/medial prefrontal cortex. Post-traumatic stress symptoms (PTSS) exist on a spectrum, and neural changes may occur beyond the diagnostic threshold of PTSD. The relationship was examined between PTSS and gray matter among combat- exposed U.S. military veterans. PTSS were assessed using the Clinician-Administered PTSD Scale (CAPS). Structural brain magnetic resonance imaging (MRI) was obtained on 28 combat veterans. Images were analyzed using voxel-based morphometry, and regressed against the total CAPS score and trauma load. Group contrast revealed smaller subgenual (sg) anterior cingulate (ACC), caudate, hypothalamus, left insula, left middle temporal gyrus (MTG), and right medial frontal gyrus (MFG) in the PTSD group. PTSS are associated with abnormalities in limbic structures that may underlie the pathophysiology of PTSD. These abnormalities were reported to exist on a continuum with PTSS, beyond a diagnosis of PTSD [13].
In our clinical experience with tinnitus patients diagnosed to have a predominantly central type severe disabling subjective idiopathic tinnitus (SIT), hypermetabolic alterations in the basal ganglia have been identified with functional brain nuclear medicine SPECT imaging since 1991 [14], PET since 2000 [15], and electrophysiology quantitative electroencephalography (QEEG) since 2000, and QEEG/low resolution brain electromagnetic tomography (LORETA) since 2002 [14,2,15,16].
It is hypothesized that the clinical significance of the caudate hyperactivity, validated with hyper metabolism with SPECT and PET and QEEG/LORETA source localization may clinically be reflective of an interneuronal corticostriatal thalamic network for the affective emotional component of the tinnitus in all tinnitus patients particularly of the SIT type.
It is hypothesized that the clinical course of a particular type of SIT, can be viewed from the paradigm of a medical trauma, (i.e. onset of the tinnitus over which the patient has no control, and lingering PTSD. The “tinnitus”, i.e. the sensory component of the aberrant auditory complaint, is perceived as a stressor over which the patient has no control. As such the patient is sensitized by the initial onset of the sensory component of the tinnitus with an accompanying severe anxiety. The sensory component of the tinnitus, the aberrant auditory sensation, predominates in the initial clinical course of the tinnitus complaint. The intensity of both the sensory and affect components fluctuate in intensity and in the clinical significance to the patient. Gradually in some SIT patients it may be superseded by and fluctuate in intensity and annoyance with the affective behavioral emotional component, which may become predominant and most significant to the patient. The new diagnosis of “tinnitus” represents a constant irritant, a loss of his/her “perfect” self. A constant reminder of an “imperfection”. The patient becomes hyper vigilant for any irritant that may increase the intensity of sensory component of the tinnitus, e.g. stimulant wine, caffeine, noise exposure. The treatment recommendations of avoidance of noise, ear protection, pharmacologic intervention of anxiolytic/antidepressant medication, instrumentation - all - evoke a memory and ultimate fear of the tinnitus. The quality of life of the patient has been compromised, confusion develops when alone, particularly as reported with interference in sleep, and cognitive function. The “tinnitus” becomes SIT, i.e. a “severe” and constant injury to the patient. The level of awareness and hypervigilance of the patient is translated to an overactivity of the sympathetic nervous system [9]. There is an activation of the classical “fight or flight” syndrome and resultant upset in the homeostasis of brain function [17].
The caudate has been identified as the neuroanatomic substrate for emotional trauma. As such when correlated with the clinical course of the SIT, its significance for the presentation of SIT in this patient may be viewed from the paradigm of a medical trauma (i.e. onset of tinnitus, perceived as a severe stressor over which the patient has no control) and a lingering Post-Traumatic Stress Disorder, which is other than from a physical insult which is usual in TBI brain trauma patients. IN this patient no conclusion has been made for PTSD. To be determined by the clinical course of the tinnitus and consulting Psychiatrist.
E. Comorbidities:
The complexity (ies) of the tinnitus for this patient both for tinnitus diagnosis and treatment was compounded by the presence of comorbidities and reflected in the initial QEEG data. The QEEG data is not a diagnostic tool for tinnitus. The QEEG data when correlated with the clinical history, physical examination and clinical course, provided adjunct information for an increase in accuracy for the tinnitus diagnosis, and translation of what is known of the pathophysiology of the pattern(s) of brain wave activity(ies) for sensations in general and specifically tinnitus, the perception of an aberrant auditory sensation.
The sequential QEEG data reflected the results of the initial combined treatment protocol, a tinnitus targeted therapy (REF Palliation 2004), based on a rationale of translation of principles of sensory physiology. Specifically, the basic principle that all sensations have components: 1) sensory, the sensation, 2) affect, the emotional behavioral component; 3) and psychomotor, proprioceptive component [18].
Additional components have been identified and include memory, and chronicity (LP). The tinnitus evaluation identified the parameters of tinnitus identification [19] i.e. the characteristics of the SIT. The cochleovestibular evaluation assessed peripheral ear function.
The Qeeg/Loreta identified what is and is not known of brain function(s) based on patterns of brain wave activity in multiple neuroanatomic substrates regions of interest (ROIs). Factors influencing the clinical course of the SIT were identified and treated in the ear, fluctuation in of aeration of the middle ears bilateral, secondary endolymphatic hydrops rt, and in brain, presumed intracranial hypertension.
F. Idiopathic intracranial hypertension:
The diagnosis of intracranial hypertension was presumed from the clinical history of head trauma associated with the ski accident 01/2014 and alteration of the complaint of ear blockage rt and tinnitus intensity with position change. In support of this presumption is the reported position influenced alteration in tinnitus intensity and ear blockage i.e. headache and increase in tinnitus intensity and ear blockage rt in the supine position, and reduction in the erect position. The headache 12/12/14 was reported frontal, overlying eye left and nuchal; and 1/19/15 occasional frontal and reduction nuchal in location.
G. Fear/Misophonia:
The SIT patient in general is fearful of the present and his future, particularly when informed that there is no cure for the tinnitus at this time. Initial concern was expressed for a return to excellence in sports, social and business activities. When approaching sleep, there was an anxiety reported for an increase in tinnitus intensity. The diagnosis misophonia, fear of tinnitus, is to be considered.
H. Medical significance
Medical significance of the tinnitus ear rt and occasional ear lt at this time includes the following considerations:
a) The tinnitus as described, clinically is considered to be a reflection of a gradual increasing sensorineural hearing loss.
b) A bilateral sensorineural hearing loss, rt>lt. Has been identified at this time predominantly in the ultrahigh frequencies 10-16 kHz. Clinically it may be related to the head trauma reported 01/2014.
c) Neurology consultation recommended for evaluation and treatment of complaint(s) that persist related to the head trauma, and cervical disc e.g. headache, depression and CNS disease clinically manifested by complaints of sensory disorders, and memory.
d) The clinical course of the tinnitus as described may be a “soft” sign of PTSD. Its significance to be determined by the clinical course of the patient. Conservative treatment recommended at this time.
I. Treatment plans:
Plan: Attempt tinnitus relief/maintenance hearing:
Initial plan 12/2014 - end 05/2015 was emphasis on the sensory and affect component of the tinnitus:
General rules of noise protection, diet