Research Article - Current Pediatric Research (2022) Volume 26, Issue 2
Electroencephalogram and cerebral hemodynamic monitoring in pediatric patients with sepsis and septic shock.
Hanan M Ibrahim Youssef1, Mervat Gamal El-Din Mansour1, Iman Ali Abdel Hamid Elagouza2, Hossam Moussa El Sayed Sakr3, Ahmed Rezk Ahmed Rezk1, Samuel Noshy Azer Beshay1*
1Department of Pediatrics, Pediatric Critical Care Unit, Ain Shams University, Cairo, Egypt
2Department of Pediatrics, Pediatric Neurology Unit, Ain Shams University, Cairo, Egypt
3Department of Radiology, Pediatric Imaging Unit, Ain Shams University, Cairo, Egypt
- Corresponding Author:
- Samuel Noshy Azer Beshay
Department of Pediatrics, Pediatric Critical Care Unit, Ain Shams University, Cairo, Egypt
E-mail: dr_samuel@med.asu.edu.eg
Received: 24 January, 2022, Manuscript No. AAJCP-22-52217; Editor assigned: 25 January, 2022, PreQC No. AAJCP-22-52217(PQ);
Reviewed: 15 February, 2022, QC No. AAJCP-22-52217; Revised: 22 February, 2022, Manuscript No. AAJCP-22-52217(R);
Published: 28 February, 2022, DOI:10.35841/0971-9032.26.2.1250-1254.
Abstract
Introduction: Sepsis-Associated Brain Dysfunction (SABD) is a potentially fatal but treatable mental impairment induced by underlying sepsis that is related to impaired cerebral auto regulation, leading to cerebral hypo or hyper perfusion. The present study attempted to monitor the cerebral hemodynamics as well as Electro Encephalon Gram (EEG) findings in pediatric patients suffering from sepsis and septic shock. Methods: The current case-control study recruited 56 children hospitalized to the PICU with septic shock or sepsis. Trans cranial Doppler parameters (Resistive Index (RI), Plasticity Index (PI), median Velocity in Middle Cerebral Artery (mVMCA), Vasomotor Reactivity (VMR) and Cerebral Blood Flow Index (CBFi) were assessed daily, along with a daily 10-minutes EEG recording for the predominant brain electrical activity during the first four days of admission. Results: The study comprised 20 children with sepsis, 16 with septic shock and 20 age and sex matched non-septic critical children (pSOFA score 3.2 ± 2.1, 7.3 ± 3.9, and 0.5 ± 1.1 respectively). Compared to control and sepsis groups, patients with septic shock had a significant increase in PI, mVMCA, and CBFi at hours 48 and 72 of enrollment, while cerebral VMR was disturbed at hours 24 and 48? RI remained unchanged in septic shock patients. Excessive theta waves were the most prominent EEG pattern in the first 24-48 hours in septic shock patients, followed by burst suppression at hour 72. Conclusion: Most patients with septic shock experienced impaired cerebral vasomotor reactivity and EEG changes in the first two days that precede changes in PI and CBFi.
Keywords
Septic shock, Cerebrovascular autoregulation, Vasomotor reactivity, Pulsatility index, Sepsis associated brain dysfunction, Transcranial doppler.
Introduction
Sepsis-Associated Brain Dysfunction (SABD) is a diffuse brain impairment that develops in patients with infection, accompanied by a systemic inflammatory response, but with no laboratory or clinical evidence of direct infection of the brain [1,2]. According to the literature, more than 70% sepsis cases experience encephalopathy symptoms [3]. An important point is the fact that mortality directly correlates with the severity of SABD [4].
Pediatric patients who suffer from SABD need specialized treatment since the occurrence of encephalopathy with sepsis elevates mortality to 50% compared to 26% in non-encephalopathy cases [4]. There is a paucity of literature on studies of SABD in pediatric patients with sepsis and septic shock. Consequently, the present study attempted to study the cerebral hemodynamics as well as EEG findings in pediatric patients with septic shock and sepsis after being admitted to the intensive care unit compared to non-septic controls.
Materials and Methods
The present case-control study was carried out in our 32-beds (PICU) of children's hospital, Ain Shams University, Cairo, Egypt, during a 19-month period (June 2019-January 2021). The ethics committee of the faculty of medicine's ethical committee, Ain-Shams University authorized the protocol of the current study, and informed consent was provided by the participants' legal guardian/parent. Pediatric patients were eligible if they were 1-60 months old and had septic shock or sepsis in accordance with the third international criteria definitions for septic shock and sepsis with the calculation of pSOFA score [5]. Exclusion criteria were: 1) Patients beyond 60 months of age, 2) with cerebral infections, 3) known cerebral lesions (hemorrhage, stroke, tumors), 4) known to be epileptic, 5) with history of inborn errors of metabolism, and 6) with disturbed conscious level due to intoxication to drugs.
The included patients were further subdivided into three groups, according to pSOFA score, as follows: 20 patients with sepsis, 16 subjects with septic shock, and 20 healthy subjects as controls. Demographic data, including age, gender, duration of PICU stay, cause of sepsis, in addition to related microbiological outcomes, were collected. Laboratory and clinical data regarding the failure of the organ were also collected. Neurological examination was daily performed, including full unresponsiveness outline FOUR score, assessment of pupils, tone, and deep tendon reflexes with a calculation of COMFORT score in sedated patients.
Vascular hemodynamics were monitored by pulse oximetry, monitoring of non-invasive blood pressure, heart rate, Central Venous Pressure (CVP), urine output, and daily functional echocardiography was done using general electric medical echocardiographic machine (Model: SAMSUNG HM70A, SAMSUNG ELECTRONICS, Hampshire-United Kingdom) to assess Systemic Vascular Resistance Index (SVRI) and Cardiac Index (CI).
Electro Encephalo Gram (EEG) was recorded daily for four successive days in all patients while lying in a supine position, using Nihon Kohden Neurofax JE-921A portable EEG system (Nihon Kohden Corporation, Tokyo, Japan) with the placement of twenty-one electrodes in accordance with the international 10–20 system for standardized placement. Every recording lasted for 10 minutes. Standardized analysis of EEG included characterization of basic parameters: predominant frequency (beta, alpha, delta, or theta), burst suppression as well as voltage. Based on the classification of Young et al., the severity of encephalopathy is classified into five categories: 1) Normal EEG, 2) Excessive theta (low voltage, generalized waves >4 Hz but <8 Hz), 3) Predominant delta (mid to high voltage waves of 4 Hz or less), 4) Triphasic waves, and 5) Burst suppression pattern [6].
Transcranial doppler sonography was performed daily for 4 continuous days using general electric medical ultrasonography machine (Model: SAMSUNG HM70A, SAMSUNG ELECTRONICS, Hampshire-United Kingdom). A 2-MHz transducer was placed at the left side of the skull through the transtemporal acoustic approach (placed just anterior to the tragus of the ear and superior to the zygoma), where M1 portion of the middle cerebral artery (MCA) could be accessible. All patients were supine with elevated head of no more than 30o. We calculated Pulsatility Index (PI= ), resistive index (RI= , Mean Velocity in Middle Cerebral Artery (mVMCA) as well as Cerebral Blood Flow Index (CBFi= ), in which MAP indicates mean arterial pressure. Cerebral Vasomotor Reactivity (VMR) by CO2 reactivity test was performed by lowering the initial PaCO2 of each patient by at least 6 mmHg by controlled hyperventilation and calculated as follows:
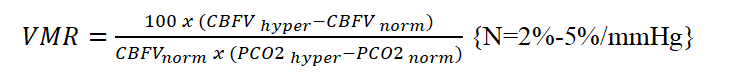
The tabulation as well as statistical analysis of data was performed utilizing Statistical Package for Social Sciences (SPSS) software, version 20 (SPSS Inc., Chicago, IL). The expression of qualitative data was in the form of and percentage (%) and frequencies (n). Quantitative data were expressed as Standard Deviation (SD) and medians. ANOVA test as well as independent t-test was utilized for comparison of quantitative variables. Fisher exact test was used to test the association between qualitative variables. Pearson correlation coefficient was utilized for correlation between quantitative variables. The level of significance was determined at p-value ≤ 0.05.
Results
This study included 56 pediatric patients (16 subjects suffering septic shock, 20 subjects who have sepsis and 20 non-septic subjects) in the age group 1-60 months (mean age 8.9 ± 9 months). Boys comprised 60% (n=34/56) and girls comprised 40% (n=22/60). Mean SOFA score at enrollment was 0.5 ± 1.1, 3.2 ± 2.1 and 7.3 ± 3.9 among the control, sepsis, and septic shock groups respectively. Mean FOUR score at time of enrollment was 14.9 ± 0.7, 11.7 ± 1.8, and 9.6 ± 1.1 among control, sepsis, and septic shock groups respectively. The primary sites of infection were pulmonary in 45 (80.3%) and extra-pulmonary in 11 (19.7%). Among the cases with pulmonary dysfunction, community acquired pneumonia was seen in 27 (48.2%), bronchiolitis was seen in 12 (21.4%) and ventilator associated pneumonia in 6 (10.7%). The extra-pulmonary sites were central line associated blood stream infection in 5 (8.9%), gastro-intestinal in 5 (8.9%), and urinary tract infection in 1 (1.7%). The mean duration of the pediatric intensive care unit stay was 10.7 days, 13.1 days, and 19.5 days among control, sepsis, and septic shock groups respectively. The overall mortality among the studied patients was 26.7%. Patients with sepsis and septic shock had higher incidence of vasopressor use and mechanical ventilation. Table 1 shows the clinical and physiological data of the studied patients.
| Physiological and clinical data of the studied groups | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Control group n=20 | Sepsis group n=20 | Septic shock group n=16 | |||||||||||
| Hour 0 | Hour 24 | Hour 48 | Hour 72 | Hour 0 | Hour 24 | Hour 48 | Hour 72 | Hour 0 | Hour 24 | Hour 48 | Hour 72 | |||
| Ventilation | Non ventilated | 8 (40) | 9 (45) | 7 (35) | 12 (60) | 4 (20) | 5 (25) | 6 (30) | 7 (35) | 1 (6.2) | 1 (6.2) | 1 (6.2) | 1 (6.2) | |
| Non-invasive ventilation | 2 (10) | 3 (15) | 4 (20) | 1 (5) | 4 (20) | 2 (10) | 2 (10) | 2 (10) | 4 (25) | 2 (12.5) | 1 (6.2) | 0 (0) | ||
| Invasive ventilation | 10 (50) | 8 (40) | 9 (45) | 7 (35) | 12 (60) | 13 (65) | 12 (60) | 11 (55) | 11 (68.8) | 13 (81.2) | 14 (87.5) | 15 (93.8) | ||
| Comfort score | Not sedated | 15 (75) | 15 (75) | 15 (75) | 15 (75) | 12 (60) | 10 (50) | 10 (50) | 12 (60) | 9 (56.2) | 7 (43.8) | 7 (46.7) | 9 (56.2) | |
| Under sedation | 1 (5) | 1 (5) | 1 (5) | 1 (5) | 3 (15) | 3 (15) | 3 (15) | 3 (15) | 1 (6.2) | 1 (6.2) | 1 (6.7) | 1 (6.2) | ||
| Adequate sedation | 3 (15) | 3 (15) | 3 (15) | 3 (15) | 5 (25) | 5 (25) | 5 (25) | 5 (25) | 5 (31.2) | 6 (37.5) | 5 (33.3) | 5 (31.2) | ||
| Over-sedation | 1 (5) | 1 (5) | 1 (5) | 1 (5) | 0 (0) | 2 (10) | 2 (10) | 0 (0) | 1 (6.2) | 2 (12.5) | 2 (13.3) | 1 (6.2) | ||
| Neurological examination | Pupils | Pupil and corneal reflexes are present | 20 (100) | 20 (100) | 20 (100) | 20 (100) | 20 (100) | 20 (100) | 20 (100) | 19 (95) | 16 (100) | 16 (100) | 15 (93.7) | 14 (87.5) |
| Pupil and corneal reflexes are absent | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 1 (6.25) | 2 (12.5) | ||
| Tone | Normal tone | 20 (100) | 20 (100) | 20 (100) | 20 (100) | 20 (100) | 20 (100) | 20 (100) | 19 (95) | 16 (100) | 16 (100) | 15 (93.8) | 14 (87.5) | |
| Hypotonia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 1 (6.2) | 2 (12.5) | ||
| Deep tendon reflexes | Present | 20 (100) | 20 (100) | 20 (100) | 20 (100) | 20 (100) | 20 (100) | 20 (100) | 19 (95) | 16 (100) | 16 (100) | 15 (93.8) | 13 (81.2) | |
| Absent | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 1 (6.2) | 3 (18.8) | ||
| Hyper-reflexia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
Table 1. Physiological and clinical data of the studied groups.
Regarding transcranial doppler ultrasonography parameters (pulsatility index, resistive index, mean velocity in the MCA, and cerebral blood flow index) during the time of enrollment, the present study did not detect any substantial differences among the control, sepsis, and septic shock groups (Table 2).
| Transcranial ultrasonographic and functional echocardiographic data of the studied groups | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Control group n=20 | Sepsis group n=20 | Septic shock group n=16 | |||||||||
| Hour 0 | Hour 24 | Hour 48 | Hour 72 | Hour 0 | Hour 24 | Hour 48 | Hour 72 | Hour 0 | Hour 24 | Hour 48 | Hour 72 | |
| Cardiac index (L/min/m2) | 4.40 ± 1.6 | 3.88 ± 1.1 | 3.80 ± 1.5 | 3.53 ± 1.2 | 4.36 ± 2.2 | 4.34 ± 1.7 | 4.69 ± 2.1 | 3.71 ± 1.7 | 2.90 ± 1.9 | 2.86 ± 1.6 | 3.12 ± 2.0 | 3.1 ± 1.8 |
| SVRI (dynes/sec/ cm5/m2) | 1.9 ± 0.7 | 1.9 ± 0.7 | 2.1 ± 1 | 2.1 ± 0.9 | 2 ± 1 | 1.8 ± 0.9 | 1.7 ± 0.8 | 2.1 ± 0.9 | 3.8 ± 3.2 | 2.9 ± 1.8 | 2.5 ± 1.6 | 3 ± 2.8 |
| PI | 1.1 ± 0.2 | 1 ± 0.2 | 1 ± 0.1 | 0.9 ± 0.2 | 1.1 ± 0.3 | 0.9 ± 0.3 | 1 ± 0.2 | 0.9 ± 0.2 | 1 ± 0.6 | 1.1 ± 0.9 | 1.1 ± 0.5 | 1.1 ± 0.5 |
| RI | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.3 |
| mVMCA (cm/s) | 42.5 ± 10.9 | 41.6 ± 11 | 39.4 ± 9.1 | 41.2 ± 15.2 | 40.8 ± 10.4 | 39.6 ± 9.8 | 39.9 ± 10.3 | 45 ± 15.6 | 48.5 ± 15.2 | 44.3 ± 17.1 | 47.2 ± 17.8 | 48.4 ± 15 |
| CBFi | 593.1 ± 117 | 562.5 ± 78 | 542.5 ± 59.9 | 557.8 ± 52.9 | 564.5 ± 113 | 600.3 ± 120.7 | 532 ± 78.7 | 532.4 ± 70.3 | 612.2 ± 151.5 | 535.9 ± 140.3 | 536.1 ± 106.4 | 561.9 ± 196.1 |
| Cerebral vasomotor reactivity | 2.7 ± 1.2 | 2.3 ± 0.6 | 2.6 ± 1.6 | 2.7 ± 2 | 2.5 ± 1.5 | 2.7 ± 1.5 | 3.1 ± 1.9 | 2.8 ± 1.8 | 2.5 ± 2.7 | 2.7 ± 2.6 | 1.6 ± 1.3 | 2.2 ± 2 |
Table 2. Transcranial ultrasonographic and functional echocardiographic data of the studied groups. SVRI: Systemic Vascular Resistive Index; PI: Pulsatility Index; RI: Resistive Index; Mvmca: Mean Velocity in Middle Cerebral Artery; CBFi: Cerebral Blood Flow Index.
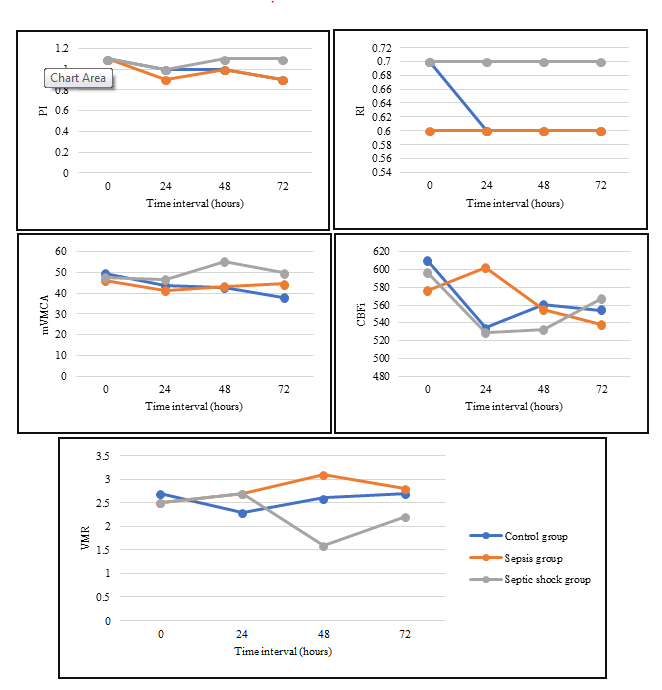
However, pulsatility index was decreased at hour 24 of enrolment in the three studied groups compared to initial PI value, with subsequent increase at hour 48, with the septic group patients having higher PI values compared to other two groups. mVMCA and CBFi were initially decreased, followed by a remarkable increase in the septic shock group at hour 48 and 72 of enrollment, respectively (Figure 1).
Also, resistive index remained unchanged in both sepsis and septic shock groups along the whole-time interval of the study in Figure 1. The cerebral vasomotor reactivity evidenced by CO2 reactivity was noticeably disturbed at hour 24-48 of enrolment in septic shock patients compared to control patients (Figure 1).
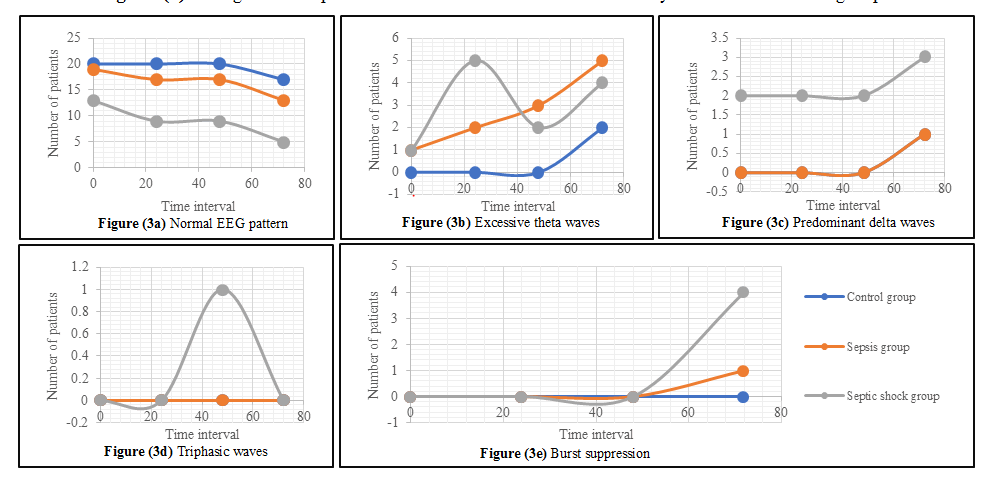
Regarding EEG patterns, the present study found that excessive theta waves were the most prominent pattern change in the first 24-48 hours. This was followed by burst suppression pattern at hour 72 in septic shock patients (Figure 2). Mortality among those with burst suppression was 100%.
Discussion
The correlation between septic shock and severe sepsis as well as elevated cerebral vascular resistance as evaluated by TCD calculated RI and PI, has previously been reported in adult patients [7]. There is a paucity of studies on SABD in pediatric patients with septic shock and sepsis. Consequently, the present study aimed to study the cerebral hemodynamics as well as EEG findings in pediatric patients after being admitted to the intensive care unit with sepsis and septic shock compared to non-septic controls.
The present study showed that most patients had a high PI at some point during enrollment. Knowing that increased PI is associated with increased cerebral vascular resistance, we suspect that most patients showed cerebral microcirculation impairment. Also, patients with high PI showed a lower mean mVMCA and CBFi compared to those with lower PI. This finding potentially resulting in a decrease in CBF in these patients. Thus, patients with septic shock may be exposed to a decrease in CBF because of increased cerebrovascular resistance and disturbed cerebral auto regulatory mechanisms. The significant PI elevation was observed in many other studies [8-12].
Although the elevated PI indicates an increase in cerebrovascular resistance that may promote a decrease in Cerebral Blood Flow Velocity (CBFV), we noticed that the PI increase, evident 48 hours after the enrollment, was associated with increases mean CBFV. A reasonable explanation for this phenomenon is that vasoconstriction triggers an increase in cardiac output with a disproportional increase in systolic CBFV and a decrease in diastolic CBFV, ultimately leading to a final increase in mean CBFV [8].
Cerebral vasomotor reactivity evidenced by CO2 reactivity was significantly disturbed at hour 24-48 of enrollment in septic shock patients compared to control patients. This is of clinical importance, because in patients with impaired cerebral auto regulation, the cerebral perfusion pressure needs to be closely controlled to avoid cerebral ischemia due to low cerebral perfusion pressures. Therefore, our study suggests a closely controlled cerebral perfusion pressure in septic shock patients during the first 2 days. This is consistent with study by Schramm et al. [13] showing a disturbed cerebral auto regulation in patients with severe sepsis and septic shock during the first 2 days of sepsis.
Our study found that excessive theta waves in EEG were the most prominent pattern change followed by burst suppression pattern in septic shock patients. Mortality among patients with burst suppression was 100%. These findings are supported by a systematic review by Hosokawa et al. [14] which showed that the incidence of EEG abnormalities in sepsis ranged from 12% to 100%. Another study showed that the ICU mortality of patients with burst suppression was double that of patients without burst suppression, and as high as the total hospital mortality [15].
Conclusion
Most patients with septic shock experienced impaired cerebral vasomotor reactivity and EEG changes in the first two days that precede changes in PI and CBFi.
Abbreviations
ANOVA: Analysis Of Variance; CBFi: Cerebral Blood Flow Index; CBFV: Cerebral Blood Flow Velocity; CBFV hyper: Cerebral Blood Flow During Hypercapnia; CBFV norm: Cerebral Blood Flow During Normocapnia; CI: Cardiac Index; CO2: Carbon Dioxide; CVP: Central Venous Pressure; EEG: Electroencephalogram; FOUR: Full Outline of Unresponsiveness; mVMCA: Mean Velocity In The Middle Cerebral Artery; PI: Pulsatility Index; PICU: Pediatric Intensive Care; pSOFA: Pediatric Sequential Organ Failure Assessment; RI: Resistive Index; SABD: Sepsis-Associated Brain Dysfunction; SD: Standard Deviation; SVRI: Systemic Vascular Resistive Index; VMR: Vasomotor Reactivity.
Acknowledgement
The authors thank the participants who were all contributed samples to the study. I thank Professor Hanan M Ibrahim and Professor Mervat Gamal for their support and my mentor Rezk for his continuous help and support.
Data presented in this manuscript are part of a doctoral thesis presented by Samuel Noshy (Assistant lecturer, Department of Pediatrics, Ain-Shams University, Cairo, Egypt) to the Faculty of Medicine, Ain-Shams University and Cairo, Egypt.
References
- Pantzaris ND, Platanaki C, Tsiotsios K, et al. The use of electroencephalography in patients with sepsis: A review of the literature. J Transl Int Med 2021; 9(1): 12. [Crossref][Google Scholar] [Indexed]
- Bodareva NV, Zabrodskaya YM, Savvina IA. To the question of septic encephalopathy: Clinical and morphological parallels. J Complement Med Res 2021; 12(2): 52-60. [Crossref][Google Scholar]
- Rudnov VA. Kulabukhov VV. The evolution of ideas about sepsis: The story continues. Infection in Surgery 2015; 13(2): 6-10. [Crossref][Google Scholar] [Indexed]
- Kaur J, Singhi P, Singhi S, et al. Neurodevelopmental and behavioral outcomes in children with sepsis-associated encephalopathy admitted to pediatric intensive care unit: A prospective case control study. J Child Neurol 2016; 31(6): 683-90. [Crossref][Google Scholar] [Indexed]
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 2016; 315(8): 801-10. [Crossref][Google Scholar] [Indexed]
- Young GB, Bolton CF, Archibald YM, et al. The electroencephalogram in sepsis-associated encephalopathy. J Clin Neurophysiol 1992; 9(1): 145-52. [Crossref][Google Scholar] [Indexed]
- de Azevedo DS, Salinet AS, de Lima Oliveira M, et al. Cerebral hemodynamics in sepsis assessed by transcranial doppler: A systematic review and meta-analysis. J Clin Monit Comput 2017; 31(6): 1123-32. [Crossref][Google Scholar] [Indexed]
- Pierrakos C, Antoine A, Velissaris D, et al. Transcranial doppler assessment of cerebral perfusion in critically ill septic patients: A pilot study. Ann intensive care 2013; 3(1): 1-7. [Crossref][Google Scholar] [Indexed]
- Fülesdi B, Szatmári S, Antek C, Fülep Z, et al. Cerebral vasoreactivity to acetazolamide is not impaired in patients with severe sepsis. J Crit Care 2012; 27(4): 337-43. [Crossref][Google Scholar] [Indexed]
- Szatmári S, Végh T, Csomós Á, et al. Impaired cerebrovascular reactivity in sepsis-associated encephalopathy studied by acetazolamide test. Critical care 2010; 14(2): 1-7. [Crossref][Google Scholar] [Indexed]
- Zidan DH, Helmy TA, Taha A. Role of transcranial doppler and FOUR score in assessment of sepsis-associated encephalopathy. Res Opinion Anesth Intens Care 2017; 4(4): 209. [Crossref][Google Scholar] [Indexed]
- Algebaly H, ElSherbini S, Galal A, et al. Transcranial doppler can predict development and outcome of sepsis-associated encephalopathy in pediatrics with severe sepsis or septic shock. Frontiers Pediatr 2020; 8: 450. [Crossref][Google Scholar] [Indexed]
- Schramm P, Klein KU, Falkenberg L, et al. Impaired cerebrovascular auto regulation in patients with severe sepsis and sepsis-associated delirium. Critical care 2012; 16(5): 1-8. [Crossref][Google Scholar] [Indexed]
- Hosokawa K, Gaspard N, Su F, et al. Clinical neurophysiological assessment of sepsis-associated brain dysfunction: A systematic review. Critical Care 2014; 18(6): 1-2. [Crossref][Google Scholar] [Indexed]
- Watson PL, Shintani AK, Tyson R, et al. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med 2008; 36(12): 3171. [Crossref][Google Scholar] [Indexed]