Review Article - Environmental Risk Assessment and Remediation (2017) Volume 1, Issue 1
Efficiency of invertebrate animals for risk assessment and biomonitoring of hazardous contaminants in aquatic ecosystem, a review and status report.
AbdAllah AT*
Zoology Department, Faculty of Science, AlAzhar University, Egypt
- *Corresponding Author:
- AbdAllah AT
Biology Department, Faculty of Science
Jazan University, KSA
Saudi Arabia
Tel: 00966542677447
E-mail: Abd_allaht@hotmail.com
Accepted date: January 03, 2017
Citation: AbdAllah AT. Efficiency of invertebrate animals for risk assessment and biomonitoring of hazardous contaminants in aquatic ecosystem, a review and status report. Environ risk assess remediat. 2016;1(1):13-18.
Abstract
Invertebrate animals exhibited successful detoxification and storage of hazardous inorganic contaminants. Moreover, waterflea Daphnia sp., other crustacean arthropods and molluscan species were implied as sentinel organisms for aquatic contaminants. Benthic invertebrates might be used as biological monitors for heavy metal pollution. They spend most of their lives at the contaminated site. They can determine the concentration of existed heavy metal pollution. A variation in the efficiency of heavy metal accumulation was shown between different invertebrate groups. Molluscan animals specially gastropods showed the greatest capability to bioaccumulate heavy metals within their digestive cells. They acquired higher bioaccumulation factor relative to arthropods and annelids. At the cellular level, heavy metals might be stored after detoxification as granules of different shapes and sizes or as lipid droplets. The present review discusses the capability of different invertebrate groups to detoxify and store inorganic contaminants and their efficiency as possible biomonitor organisms for aquatic ecosystem.
Keywords
Invertebrates, risk assessments, hazardous contaminants, biomonitoring, aquatic ecosystem.
Introduction
Rapid growth nowadays in the economy and increasing human population has led to the increase of land use, energy, agricultural products, raw materials and investment. Uncontrolled disposal of inorganic and organic waste materials in the aquatic ecosystem has resulted in significant losses of biodiversity of inhabitant invertebrates [1,2]. Heavy metals are more hazardous pollutants than organic contaminants as they are non-degradable. Moreover, their toxic effect can persist for a longtime [3,4]. Hazardous contaminants have either direct or indirect effect on human health [5]. They might show severe impact on animal life [6-9]. If the metal concentration exceeds a threshold level, the toxic signs start to be manifested in the exposed organism. Variation in acute and chronic toxicity was shown among vertebrates and invertebrate animals. Caddis flies were studied as biomonitors for threshold metal toxicity affecting stream benthos [10].
Several studies reported the possible use of invertebrates as sentinel organisms for heavy metals, inorganic and some organic contaminants [2,11-14]. Cladoceran Daphnia is very sensitive for aquatic pollutants [15,16]. It is commonly used as bioindicator for hazardous contaminants at biomonitoring stations.
Good water quality was defined by Kindt [17] as the lack of toxic substances, garbage, industrial wastes, sewage sludge, radioactive wastes and oil. Nkwoji, et al. [18] reported the use of diversity and abundance of macrobenthos as bioindicators for water quality because of their variable response to hazardous contaminants. Hadley [5] stated that the pronounced decrease or increase in population density of macroinvertebrates can be used as bioindicators for poor water quality.
Entry of heavy metals into animal cells
Heavy metals are more hazardous pollutants than other contaminants as they are non-degradable. Moreover, their toxic effect can persist for a longtime [3]. Severe toxic effect can be observed in the exposed organisms after the concentration of hazardous contaminant attained a threshold level. Living organisms vary in their tolerance limits based on their sensitivity to various metals [11].
Uptake of heavy metals from the inhabitant water or intake via feeding on algae or predation of small animals are the main routes of entry of metals into animal cells [1,11,19]. Only dissolved free metal ions are the available heavy metals to uptake into animal cells [20]. Mechanism of detoxification is described by previous studies for entered metals binding them with special amino acid called metallothionin (a sulphhydryl-rich protein with low molecular weight) forming granules or electronic dense or translucent vesicles of various sizes [1,21,12,13]. The amount of heavy metals concentrated in the animal tissue is the difference between the entered heavy metal and released. The concentration of a substance within the accumulator organism is the difference between the amount taken in and the amount released [4].
Trace metals entry across cytoplasmic membrane was proposed through four primary routes; carrier-mediated transport Where the metals continue to enter the cell passively whatever, the cellular metal concentration is higher than outside the cell, protein channels through hydrophilic core proteins, passive diffusion for lipid soluble metals or endocytosis where the cell membrane engulf metal associated particles transferring into cellular vesicles [12,22-24]. Various factors affect heavy metal uptake from the inhabitant aquatic ecosystem such as membrane permeability, pH, water temperature, water hardness, and acid radical of the metal salt [4].
Biomonitoring aquatic contaminants
Monitoring heavy metal pollution using sensitive chemical instruments is not valuable. Heavy metals exist in aquatic environment as complexes or free ions. Only free ions are available for living organisms. Moreover, if the analysis with those instruments resulted in nonhazardous concentrations of metal pollutants, the metal concentrations can be transferred and multiplied across the food chain from one consumer to the other [25]. Simkiss et al. [11] suggested the roles for efficient biological monitor. The selected organism should has the ability to detoxify the contaminant turning it into non-harmful compound and should store them several folds of the inhabitant water or sediments. Also, stored contaminant should not affect the healthy status of organism. The selected organism should have a life cycle of at least on year to allow the following of contaminant concentration within its tissue throughout different seasons. It should be of big size to allow analysis of soft tissue to determine the concentration of different contaminants.
Some invertebrate organisms were introduced as biomonitors for the aquatic habitats [15,16,20,25,26]. Mollusks are successfully used as biomonitors [15,26-28]. They have the ability to detoxify metal pollutants and storing them within some organelles of their tissues several folds of the surrounding habitats, so that a bioaccumulation factor can be determined for each metal [9,29]. Digestive gland in mollusks was suggested to be the site of metal storage and the production of metal granules [13].
Benthic molluscs
Molluscs proved successful capability of storage of inorganic and organic contaminants [9,2,30]. Dauvin [31,32] used macrobenthic invertebrates as indicators for the health of estuary ecosystem after oil spills. He reported a change in amphipod/annelid ratio after the oil spills. Scallop showed high bioaccumulation of lead at its soft tissue [33].
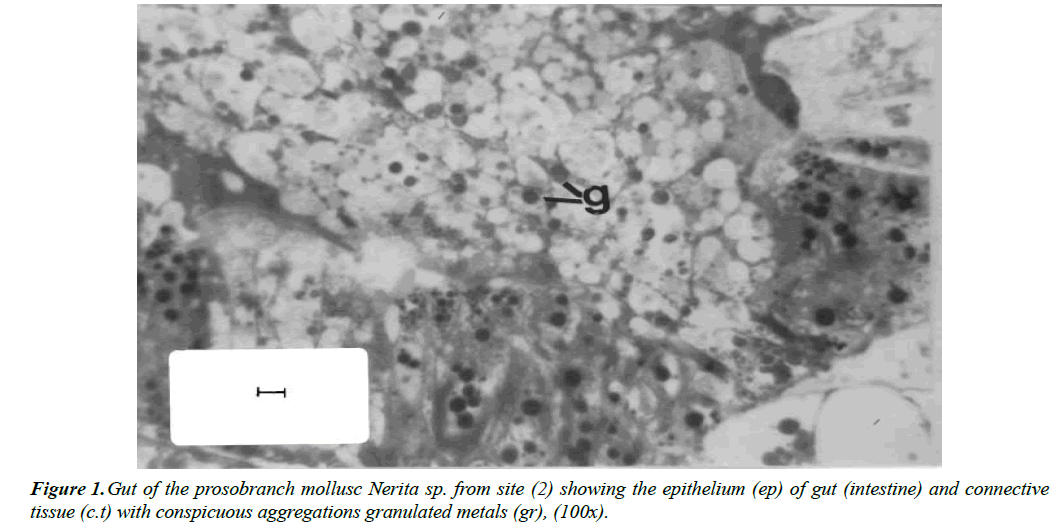
AbdAllah and Moustafa [13] found that light structure of digestive gland of the marine prosobranch snails Nerita sp. collected from both polluted and relatively clean sites almost showed the same regular histological features. However, some digestive cells of snails collected from polluted site with highly recorded levels of heavy metals exhibit deeply stained spherical granules of varying size (Figure 1).
Annelid species
Errant Polychaete Nereis succinea exhibited capability for detoxification and storage of lead, cadmium, zinc, copper, manganese and chromium in their soft tissues hundred times that of the inhabitant water [34]. Bioaccumulation factor that calculated as a ratio between soft tissue and water metal concentration showed high annual means for bioaccumulation ranked as Cu (1023.09 ± 816.55)>Cd (655.48 ± 540.46)>Mn (646.93 ± 413.44)>Fe (371.19 ± 155.46)>Cr (360.59 ± 406.87)>Pb (162.97 ± 118.03).
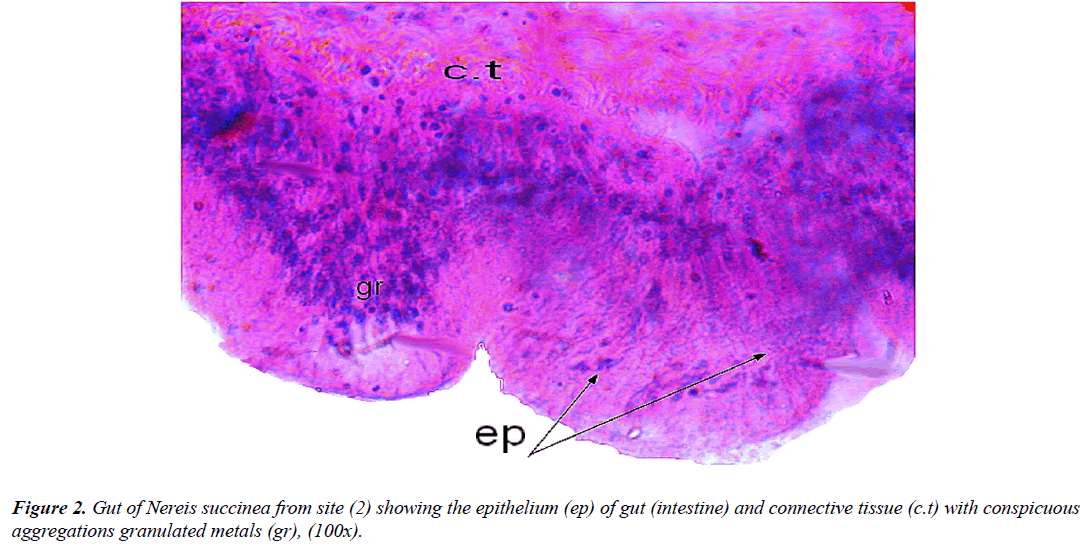
Histological examination of their soft tissues (Figure 2) displayed regular light structure of gut as those inhabiting control non contaminated areas, while those inhabiting polluted areas showed the presence of granules on some gut tissues [29,34].
Moreover, as an endobenthic species, Nereis is particularly exposed to pollutants since sediments are the main reservoir for a number of chemicals. The body size and concentrations of the energy reserves were consistently lower than in worms from a reference site that might be the consequence of the physiological cost needed to cope with the presence of toxicants [35].
Arthropod animals
The freshwater crayfish Procambarus clarki accumulated heavy metals several folds of the surrounding [36]. Mangrove crab Metopograpsus messor showed bioaccumulation of lead, copper, cadmium and zinc hundred folds of the surrounding. Water flea Daphnia was successfully used as sentinel organism for freshwater aquatic contaminants [3]. The presence of aquatic contaminant at hazardous concentration resulted in mortality of the water flea.
Zooplankton
Zooplankton organisms are important elements of the aquatic food chain. They are intermediary link in the food chain and can transfer energy from phytoplankton as primary producers to the larger invertebrates or fish [37]. Spatial distribution of zooplankton, abundance and body size were used as bioindicator for water quality at marine habitat. Presence of predator, change of water quality criteria are among the factors that control zooplankton distribution [38]. Parmer et al. [39] reported the zooplankton can help to evaluate the level of water pollution and other environmental stressors. The copepod Acartia tonsa Dana was reported by Bianchi et al. [40] in the Lagoon of Venice as an indicator species in highly eutrophic areas. Raut and Shembekar [41] reported 19 zooplankton bioindicator species including 8 rotifers, 4 cladoceran, 6 copepodes and 1 ostracode at Borna (Chandapur) Dam, near Parli, India.
Meiofauna
Meiofauna exist all life span at it’s a limited space. Therefore, they can be used as bioindicator for water quality and contamination status of their inhabiting area. Foraminifera and the metazoan nematode species were used as indicator for health status of aquatic ecosystem [42,43]. Murray [44] and Dijkstra et al. [45] used the protozoan Foraminifera assemblages as bioindicators for heavy metals and persistent organic pollutants (POP). Murray [44] found that water criteria such as salinity, dissolved oxygen, water temperature and nutrient availability affect for aminifera distribution, Bervoets et al. [46] used the two benthic invertebrates; Chironomus and Tubificid worms as predictors for ecological effects in aquatic ecosystem.
Helminth parasites
Several literatures highlighted the capability of helminth parasites to be used as bioindicator for water quality assessment. Increase of number of helminth genera and abundance of different species in the aquatic ecosystem gives an indication of quite healthy ecosystem. Nematodes showed resistance to hazardous pollutants as they were isolated from vertebrate animals inhabiting aquatic contaminated ecosystem [30,47]. Acanthocephala inhabiting fish intestine can accumulate heavy metals several folds that of their host tissues in ratio higher than that of the zebra mussel Dressina polymorpha [48]. He explained that the accumulation of metals in Acanthocephala is not a result of slow accumulation but takes place via rapid uptake process till a steady state. Vidal-Martínez et al. [49] found that polyaromatic hydrocarbons of high molecular weight (PAHH) might affect the occurrence of larval Cestode Oncomegas wageneri in the Gulf of Mexico.
Heavy metals interaction
Heavy metals, organic or inorganic contaminants occur normally combined in the aquatic habitats. Metals naturally exist in variable ratios with each other and with organic or inorganic contaminants depending on different discharge sources [50]. Wong [51] recommended the use of metal mixtures for both chronic and acute toxicity studies rather than single metal solutions, as they provide more valuable and realistic information about the nature of heavy metal toxicities in the aquatic ecosystem. Previous investigations on the toxicity of combined metals showed that the interaction of lead and copper has synergistic effect and were more toxic than single metals [9,52].
Cellular basis of Bioaccumulation of heavy metals
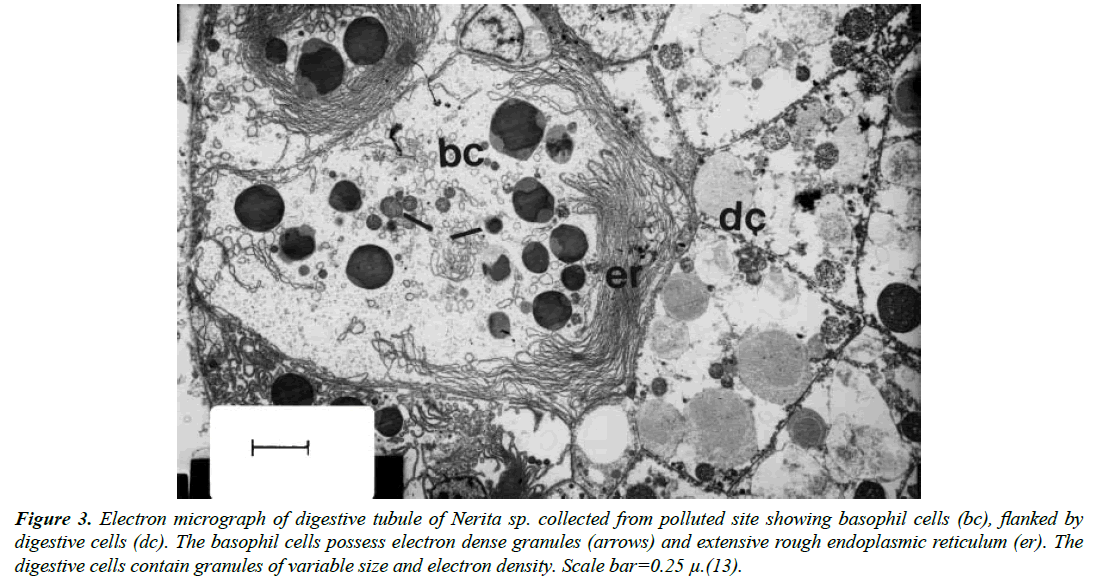
On the cellular level, basophil cells from snail live at contaminated and relatively clean sites (Figure 3) are pyramidal in shape containing a system of Golgi bodies and rough endoplasmic reticulum in the middle and basal regions. The digestive cells contain vacuoles and granules of varying in shapes, sizes and electron density (Figure 3). Copper and cadmium are detoxified after cellular uptake. Those metals were shown to bind to special amino acid forming metallothionin that is nontoxic to the living cell. Detoxified metals were stored within the lysosomes (Figure 3) in the form of electron lucent or electron dense vesicles of various shapes and sizes [1,9,13,21]. Exposure of freshwater prosobranch snails to less toxic concentrations of lead chlorides (Fig 4) showed regular structure of basophil and digestive cells. Lead was shown to be stored in the form of granules or lipid droplets [12].
Figure 3. Electron micrograph of digestive tubule of Nerita sp. collected from polluted site showing basophil cells (bc), flanked by digestive cells (dc). The basophil cells possess electron dense granules (arrows) and extensive rough endoplasmic reticulum (er). The digestive cells contain granules of variable size and electron density. Scale bar=0.25 μ.(13).
Conclusion
Many of the contaminants enter the aquatic ecosystems including lead, mercury, pesticides, and herbicides as a result of uncontrolled discharge of agricultural and industrial wastes. Variable toxic effects was shown on living organisms. They can reduce reproductive activity, inhibit growth and development, and even cause death. Filter feeders such as mussels, clams and other organisms higher in the food chain are also affected by the presence of hazardous contaminants. Most marine invertebrates possess detoxification mechanism turning the pollutants nontoxic and bioaccumulate them within their tissues several folds of the surroundings. Increase or decrease of abundance and species richness of benthic invertebrates might be used as bioindicator for water quality of the aquatic ecosystem.
Acknowledgement
Many thanks are due to the editorial board for their kind invitation to contribute to the first volume of the Journal.
References
- AbdAllah AT. On the efficiency of some histological techniques as biomarkers for heavy metal pollution. In: Mendez JS, editor. Science, technology and education of the microscopy. An overview, Formatex Publishers, Badajoz, Spain; 2003.
- AbdAllah AT. Light structure as biomarker for heavy metal bioaccumulation and toxicity in molluscan gastropods. In: Microscopy: advances in scientific research and education. Méndez-Vilas A, editor. Formatex Publishers; 2014. 330-34 pp.
- Ravera O. What is the nature of the damage caused by the pollution load of the water due toits content of substances that can be decomposed only with difficulty, as regards the biology of water? European Federation for the Protection of Waters (EFPW). 1972;28-30.
- Ravera O. Monitoring of the aquatic environment by species accumulator of pollutants: a review. J Limnol. 2001;60:63-78.
- Hadley D. Water Quality Monitoring Using Aquatic Macroinvertebrates, What Aquatic Insects Can Tell You About the Quality of a Stream. Department of Environmental Resource Management, Lagos State University, Lagos, Nigeria; 2015.
- WHO Lead- Environmental aspects. Environmental Health Criteria, World Health Organization, Geneva; 1989. 106 p.
- WHO Environmental Health Criteria, 200. Copper. Interational Programme on Chemical safety. World Health Organization, Geneva; 1998.
- WHO Environmental Health Criteria, 221. Zinc. INCHEM. World Health Organization, Geneva; 2001.
- AbdAllah AT. Investigations on bioconcentration and toxicity of lead and copper to the freshwater prosobranch Lanistes carinatus. Malacologia. 2006;48:27-34.
- Rainbow PS, Hildrew AG, Smith BD, et al. Caddisflies as biomonitors identifying tresholds of toxic metal bioavailability that affect the stream benthos. Environ Pollut. 2012;166:196-207.
- Simkiss K, Taylor M, Mason AZ. Metal detoxification and bioaccumulation in molluscs. Marine Biological Letters. 1982;3:187-201.
- Simkiss K and Mason AZ. Metal ions: metabolic and toxic effects. Hochachka PW, editor. The Mollusca. Environmental biochemistry and physiology. Academic Press, New York & London. 1983;2:362.
- AbdAllah AT and Moustafa MA. Accumulation of lead and cadmium in the marine prosobranch Nerita saxtilis ; light and electron microscopy. Environ Pollut. 2002;116:185-91.
- Rombouts I, Beaugrand G, Artigas LF, et al. Evaluating marine ecosystem health: Case studies of indicators using direct observations and modelling methods. Ecological Indicators. 2013;353-65.
- Ravera O. Effects of heavy metals (cadmium, copper, chromium and lead) on a freshwater snail: Biomphalaria glabrata Say(Gastropoda, Prosobranchia). Malacologia. 1977;16:231-36.
- Ravera O, Beone GM, Trincherine PR, et al. Seasonal variations in metal content of two Unio pictorum mancus (Mollusca, Unionidae) populations from two lakes of different trophic state. J Limnol. 2007;66:28-39.
- Kindt JW. Solid Wastes and Marine Pollution. 1985;34-7.
- Nkwoji JA, Igbo JK, Adeleye AO, et al. Implications of bioindicators in ecological health: study of A coastal lagoon, Lagos, Nigeria. Agriculture and Biology. Journal North America. 2010; ISSN Print: 2151-7517.
- Luoma SN. Processes affecting trophic transfer and resultant effects of metals: implications for monitoring metal pollution in the sea. Ciesm Workshop Monographs. 2002; 75-8.
- Rainbow PS. Trace metal concentrations in aquatic invertebrates: Why and so what? Environmental Pollution. 2002;120:497-507.
- George SG. Subcellular accumulation and detoxification of metals., Vernberg WB, Calabrese A, Thurberg FP, Vernberg FJ, editors. Physiological mechanisms of marine pollutant toxicity. Academic press; 1982. 3-53 pp.
- Williams RJP. Physico-chemical aspects of inorganic element transfer through membranes. Philosophical Transactions of the Royal Society of London (Series B). 1981;294:57-74.
- Rainbow PS and Dallinger R. Metal uptake, regulation and excretion in freshwater invertebrates. Ecotoxicology of Metals in Invertebrates.Dallinger R, Rainbow PS, editors. Boca Raton: Lewis Publishers. 1993; 119-31 pp.
- Tessier A, Buffle J, Campbell PGC. Uptake of trace metals by aquatic organisms. Chemical and Biological Regulation of Aquatic SystemsBuffle J, De Vitre RR, editors. Lewis Publishers, Boca Raton. 1994; 197-230 pp.
- Philips DJH, Rainbow PS. Biomonitors of Trace Aquatic Contaminants. Elsevier Applied Science. 1993.
- Rainbow PS, Philips DJH. Cosmopolitan bio-monitors of trace metals. Marine Pollution Bulletin. 1993;26:593-601.
- Cossa D. A review of the use of Mytilus spp. As quantitative indicators of cadmium and mercury contamination of coastal waters. Oceanologia Acta. 1989;12:417-32.
- Rainbow PS. Molluscs as cosmopolitan biomonitors of heavy metal. The annual meeting of the Malacological Society of London, Manchester. 1994.
- AbdAllah AT, Moustafa MA, ElSheimy N, et al. On the efficiency of the marine polychaete Nereis succinea as biomonitor for heavy metal pollution in marine environment. Zone Côtière Canada 2012/ Coastal Zone Canada. 2012;9-14.
- AbdAllah AT and Haroun SH. Efficiency of bioaccumulators as biological monitors for heavy metal pollution. 29th meeting, Saudi Biological Society, Dammam. 2014.
- Dauvin JC. The fine sand Abra alba community of the Bay of Morlaix twenty years after the Amoco Cadiz oil spill. Mar Pollut Bull. 1998;36:669-76.
- Dauvin JC. Paradox of estuarine quality: benthic indicators and indices, consensus or debate for the future. Mar Pollut Bull. 2007;55:271-81.
- Marsden ID and Cranford PJ. Scallops Biology, Ecology, Aquaculture, and Fisheries. In Developments in Aquaculture and Fisheries Science. Shumway SE, Parsons GJ, editors. 2016;40:1-1196.
- Said. Ecological and biological studies on some marine annelids inhabiting the Red Sea, Egypt. M.Sc. Thesis, AlAzhar University, Assiut, Egypt. 2009.
- Durou C, BD Smith, M Romeo BS, et al. From biomarkers to population responses in Nereis diversicolor: Assessment of stress in estuarine ecosystems. Ecotoxicol Environ Saf. 2007;66:402-11.
- AbdAllah AT and El-Zawahry EI. Bioaccumulation of lead and copper in the freshwater crustacean Procambarus clarkii; spectrophotometric and biochemical studies Journal of Egyptian Academic Society for Environmental Development (D-Environmental Studies). 2001;2:93-106.
- Lee G and Stokes. An illustrated guide to Science. Chelsea House publishers. J Marine Sci. 2006.
- ObuidAllah AH, AbdAllah AT, Abu-Eldahab HM, et al. Impact of Heavy metal contamination on seasonal abundance of planktonic copepods inhabiting mangrove area in safaga, Red Sea, Egypt. Egypt J Exp Biol (Zool.). 2005;1:57-66.
- Parmer TK, Rawtani D and Agrawal YK. Bioindicators: the natural indicator of environmental pollution. Frontiers in Life Science. 2016;9:110-18.
- Bianchi F, Acri F, Aubry FB, et al. Can plankton communities be considered as bio-indicators of water quality in the Lagoon of Venice? Mar Pollut Bull. 2003;46:964-71.
- Raut KS and Shembekar VS. Manipulation of Zooplankton as Bio-Indicators of Water Quality at Borna [Chandapur] Dam Near Parli V Dist Beed Maharashtra, India. Indian Journal of Applied Research. 2015;5:587-92.
- Metcalfe WJ. Meiofauna abundance and distribution in Chesapeake: Relationships with environmental stressors, sediment toxicity and macrofauna. M.Sc. Thesis. Faculty of the school of marine science, The college of William and Mary in Virginia, Virginia, US. 2005.
- Boufahja F, Semprucci F and Beyrem H. An experimental protocol to select nematode species from an entire community using progressive sedimentary enrichment. Ecological Indicators. 2016;60:292-309.
- Murray J. Ecology and Applications of Benthic Foraminifera. Cambridge UniversityPress, New York. 2006.
- Dijkstra N, Junttila J, Skirbikk K, et al. Benthic foraminifera as bio-indicators of chemical and physical stressors in Hammerfest harbor (Northern Norway). Marine Pollution Bulletin. 2016.
- Bervoets L, De Jonge M, Blust R. Identification of threshold body burdens of metals for the protection of the aquatic ecological status using two benthic invertebrates. Environmental Pollution. 2016;210:76-84.
- Sures B. Accumulation of heavy metals by intestinal helminths in fish: an overview and perspective. Parasitology. 2003;126:53-60.
- Sures B. The use of fish parasites as bioindicators of heavy metals in aquatic ecosystems: a review. Aquatic Ecology. 2001;35:245-55.
- Vidal-Martinez, Torres-Irineo E, Romero D, et al. Environmental and anthropogenic factors affecting the probability of occurrence of Oncomegas wageneri (Cestoda: Trypanorhyncha) in the southern Gulf of Mexico. Parasites & Vectors. 2015;8:609.
- Clubb RW, Gaufin AR, Lords JL. Acute cadmium toxicity studies upon nine species of aquatic insects. Environ Res. 1975;9:332-41.
- Wong PTS. Toxicity of cadmium to freshwater microorganisms, phytoplankton, and invertebrates. In: Nriagu JO, Sprague JB, editors. Cadmium in the aquatic environment. New York: Wiley-Interscience; 1987. pp. 117-138.
- Harrahy A and Clements WH. Toxicity and bioaccumulation of heavy metals in Chironomus tentans. Environ Toxicol Chem. 1997;16:317-27.