- Biomedical Research (2005) Volume 16, Issue 1
Efficacy of a small dose of oral dexamethasone in croup
Mohammed Alshehr1*, Talal Almegamsi2, Anwar Hammdi31deP fo tnemtrapeDiatrics, College of Medicine, King Khalid University, Abha, Saudi Arabia
2haddeJ ,latipsoH tsilaicepS lasiaF gniK ,tinU ygolonomluP ,scirtaideP fo tnemtrapeD Saudi Arabia
3ahbA ,ytisrevinU dilahK gniK ,enicideM fo egelloC ygolocamrahp fo tnemtrapeD Kingdom of Saudi Arabia
- *Corresponding Author:
- Mohammed Abdullah Alshehri
Department of Pediatrics, College of Medicine
King Khalid University P.O Box 641
Abha, Saudi Arabia
e-mail: Fariss2000 ( at ) yahoo.com
Fax: 07-2297838
Mobile: 055750434
Accepted 01 January 2005
Abstract
Acute laryngotracheobronchitis (croup) is one of the most common childhood respiratory illnesses. Children with moderate to severe airway obstruction are traditionally admitted to hospital for observation and treatment. Corticosteroids are now frequently used in children with acute laryngotracheobronchitis. To assess the efficacy of a smaller dose of dexamethasone (0.15 mg/kg) as compared to the standard dose of oral dexamethasone (0.6 mg/kg) in children with croup. We performed a double-blind, randomized trial involving 72 children with acute laryngotracheobronchitis. One group of children had dexamethasone (0.6mg/kg) (group A) and the other group of children was treated with oral dexamethasone (0.15mg/kg) (group B). The severity of illness was assessed by a clinical croup score based on retractions, stridor, air entry, cyanosis and the level of consciousness. Reduction in croup score, hospitalization rate and admission and adrenaline usage were evaluated. The characteristics of both groups were a most similar at the base line, including, duration of symptoms, previous medication usage, and severity of illness. Most of the children (90%) being younger than 5 years of age. Seventy-two patients completed the study with 36 patients in each group. Twelve hours from the time of treatment, the patients in group A (0.6mg/kg oral dexamethasone) as well as the patients in group B (0.15mg/kg oral dexamethasone) had statistically significant decline in median croup score from 4.5 to 2 (p=0.01) and from 5 to 2 (p=0.01), respectively. The over all rate of hospitalization after treatment in group A (0.6 mg/kg dexa-methasone) was 15/36 children (41.6%) and in group B (0.15mg/kg dexamethasone) it was 14/36, (38.9%) and there was not statistically significant different from one another (p=0.36). The median hospital stay (hours) for group A was 28hr (12-50) and for group B was 26hr (14- 48). No statistical significant difference between the two groups was observed (t=-0.32, p=0.64). Other outcome measures were similar for the two groups. We conclude that oral dexamethasone in a dose of 0.15mg/kg is as effective as a dose of 0.6 mg/kg in relieving symptoms of acute croup in children and results in similar reduction in the croup score, the adrenaline usage and the overall hospital admission rate and the duration-stay.
Keywords
Acute laryngotracheobronchitis, dexamethasone, children, croup score
Introduction
Croup is an acute clinical syndrome characterized by inspiratory stridor, barking cough, and signs of respiratory distress due to laryngeal or tracheal obstruction [1-3]. It occurs in about 2% of preschool-aged children annually [1]. It mainly affects children aged 6-36 months, with a peak incidence at 12-24 months and with a male predominance of 3:2 [1].
The treatment of croup remains controversial and mild cases probably require no treatment other than careful observation. Although humidification had been used in the treatment of croup since the 19th century, it may not relive the symptoms. On the hand, the nebulized adrenaline has a beneficial effect but of limited duration [3-5]. The degree of obstruction in patients with croup returns to pretreatment levels within two hours [3].
In the past 40 years, there have been more than a dozen of clinical trials evaluating steroid utilization in acute laryngotracheobronchitis [6-15] with some of them noting a therapeutic benefit [6-10] and others showing no effect [11-15]. This equivocal evidence supporting the use of corticosteroids in acute laryngotracheobronchitis may be related to the methodological deficiencies in the previous studies, as outlined by Tunnssen and Feinstein [16].
Lately, the benefit of glucocotricoid therapy in patients with croup has been firmly established the results of four randomized clinical trials of intramuscular dexamethasone [17,18], oral prednisolone [19] and nebulized busonide [20].
Although there were no statistical difference in the efficacy of oral dexamethasone versus the efficacy of nebulized budesonide, with regard to hospitalization time or croup score, there was a consistent trend in favor of the oral preparation. Of the children who remained hospitalized, those who received oral dexamethasone had significantly lower mean pulse rate at 8 and 12hr, and lower respiratory rate at 8hr, than those who received aerosol budesonide, implying less work of breathing [21].
Oral dexamethasone was selected for this study because of its availability and its lower cost as compared to nebulized budesonide or intramuscular dexamethasone. In addition, it does not require face mask, tubing, or prolonged time of delivery in agit-ted child and it can be administered without the discomfort of intramuscular injection.
There is a concern that dexamethasone, with a serum half –life of 36-54 h [3,22], could have sustained effects on multiple systems and that, it may reduce the immune function. Persons exposed to varicella while taking glucocotricoid are at risk for severe varicella infection [23]. In a placebo-controlled trial of nebulized dexamethasone in children with moderate croup, Johnson et al [24] reported that bacterial trachitis developed in two chil-dren with occult neutropenia. In order to evaluate the role of oral steroids in children with croup, most of these studies had used a dose of 0.6 mg/kg. However, in patients who were treated with nebulized budesonide, a smaller does of steroid had been administered. Therefore, we hypothesized that smaller single dose of oral dexamethasone would be as effective as 0.6mg/kg in the treatment of croup and would cause less adverse effects. Hence, we designed a randomized, double-blind controlled trial to determine whether a smaller dose of oral dexamethasone (0.15mg/kg) would have similar beneficial effects as the usual dose (0.6 mg/kg), in children with croup.
Methods
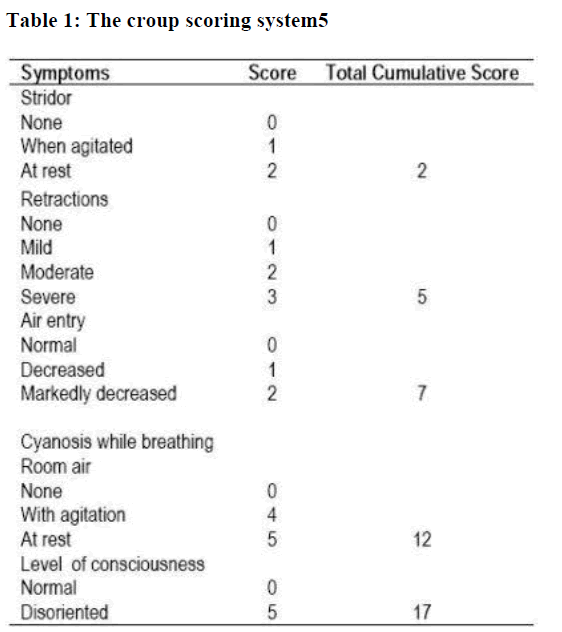
Children were enrolled in the study if they were at age of 3 months to 9 years, had been given a diagnosis of croup (defined as acute onset of inspiratory stridor associated with a “seal-like” barking cough), had persistent, moderately severe respiratory distress, defined as a croup score more than three (Table 1) [5,25] and their parents had given informed conscent to particpate in the present study. Exclusion criteria included: symptoms or signs suggesting another cause of stridor, such as epiglotitis, bacterial trachitis, or supraglottic foreign body; a history of chronic pulmonary disease, severe systemic disease, immune dysfunction, stridor or intubation for more than one month, or glucocorticoid therapy in the last four weeks before study entry. Patients were recruited in the emergency rooms and outpatient clinics from September 1998 to December 2002 in three medical institutes, Abha City, Southwestern of Saudi Arabia. To make the study-drugs indistinguishable from each other, they were packaged in opaque containers and diluted on the same amount of solution. A blocked randomization code was produced by random-number-generating software, and the code was not broken until after the study ended and all the decisions regarding data analysis were finalized. The need for further treatment with racemic epinephrine and hospitalization were based on the clinical judgment and no treatment or admission criteria were imposed. All children received mist therapy throughout the observation period. Mist was administered through a plastic hose held by the parents to the child‟s face. The co-interventions treatment was administered in a standard-ized fashion. Mist was used for a total croup score ≥2; racemic epinephrine aerosols was given for croup scores ≥4; supple-mental oxygen was used for oxygen saturation < 88%; and anti-biotics were given for causes other than croup such as otitis media. The dosage of the racemic epinephrine aerosols was 0.5 cc of 2.25% racemic epinephrine in 3.5ml of saline solution ad-ministered through an aerosol mask for 20 minutes [5].
Outcome measures
The primary and secondary outcome measures were defined before the data collection. The primary outcome measures were the change in total croup scores per 12-hour interval within and between the study groups, as well as the classification of patients as having a favorable clinical response at 12 and 24 hours after treatment. A favorable clinical response was defined as an improvement in the total croup score of ≥2 units [8]. The sec-ondary outcome measures were: 1. The requirement for cointerventions such as racemic epinephrine aerosols mist therapy or both, 2. The respiratory rate, and 3. The oxygen saturation measured by pulse oximetry. In addition, the rate of hospitalization, the intensive care unit admission and the duration of hospital stay, all were assessed.
Data analysis
Estimation of sample size was based on two-point improvement in the croup score or a return of the score to 1 or less and this was considered to be clinically important and to constitute a response. We estimated that at least 70 percent of patients as-signed to either group of treatments would have clinically impor-tant improvement. With two-sided, alpha level of 0.05 and 80 percent power, a sample containing 32 patients per group would be required. With regard to data analysis, Chi-square analysis with Yates correction was used for all dichotomous variables. The Mann-Whitney U test and the Wilcoxon matched-pair signed rank test were used for all ordinal variables and the T test and paired t test were used for interval variables. All statisti-cal tests were two-tailed test. For interval variables the results were reported as mean ± SD (standard deviation) and for ordinal variables the medians and ranges were reported [25].
Results
Eighty-four patients were enrolled in the study over four years interval from September 1998 to September 2002 and only 72 had completed the study. Other patients did not met the eligibility criteria for the study because they had croup that was too mild, did not meet the age requirement, did not meet our defini-tion of the croup, had croup that was so severe, had epiglottis, had previous upper airways disease, or/and had been treated with steroids.
Thirty-six patients were randomly assigned to receive the standard dose of 0.6mg/kg of oral dexamethasone (Group A), and the other 36 patients were assigned randomly to receive oral dexamethasone at a dose of 0.15 mg/kg (Group B).
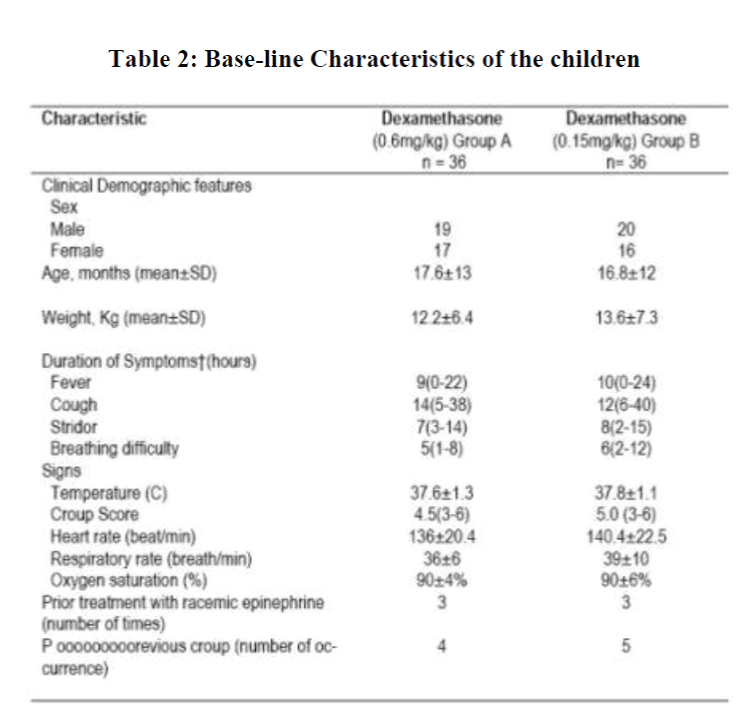
At enrollment, the two groups were almost identical regarding age, sex, duration of symptoms, previous medication usage, and severity of illness (Table 2). The children age range was 6 to 160 months with 90% being younger than 6 years of age.
A chest or lateral neck roentgenogram (or both) was obtained on nine patients in group A and ten patients in group B. None of these patients had any roentogenographic evidence of pneumonia or epiglottis at the time of study entry. However, all had subglottic narrowing on their roentogenograms. The total croup score was the same in both baseline observations.
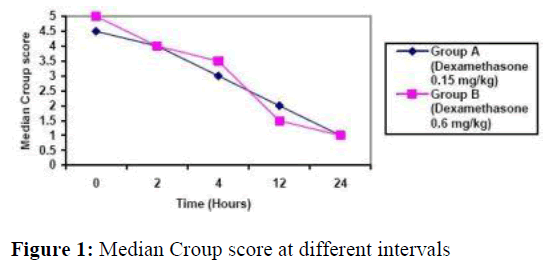
Figure 1, demonstrates decline in median croup score in both groups at different intervals. Twelve hours after treatment those who received 0.6mg/kg oral dexamethasone had significant improvement in their total croup score (Figure 1) from 4.5 to 2 (range3-6 and 1-4, respectively; Z score = -3.22. p =0.001. Wil-coxon matched-pair signed-rank test). Patients who received 0.15mg/kg oral dexamethasone had also significant improve-ment in their total croup score from 5 to 1.5 (range 3-6 and 0-3, respectively, Z score = -3.45, p= 0.001) as shown in figure 1.
At 12 hours after treatment, group A (0.6 mg/kg oral dexamethasone) total score was comparable to group B‟s score (0.15 dexamethasone) (Z score= -1.12 p= 0.15, Mann-Whitney U test).
By 24 hours, group A‟s ( 0.6 mg/kg oral dexamethasone) total croup score was 1.0 (range 0- 3) and group B‟s (0.15 mg/kg oral dexamethasone) score was 1.0 (range 0-3.5; Z score= -1.42, p=0.24, Mann-Whitney U test).
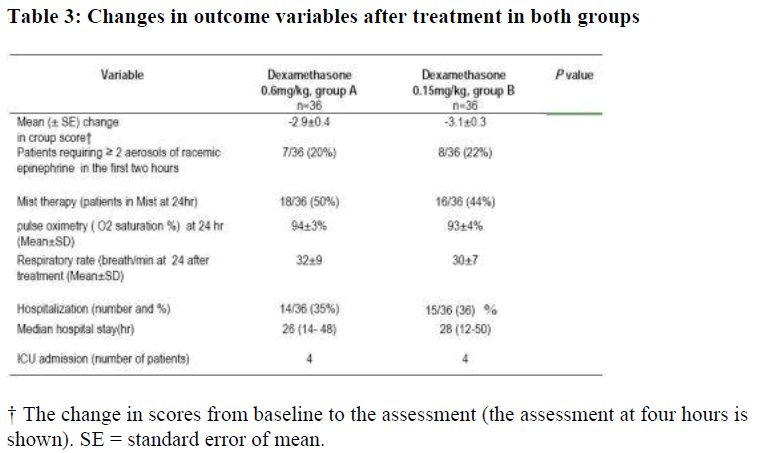
Most of the improvement in the total croup score was seen in the diminution of the retractions and stridor 24-hour after the treatment. Group A (0.6 mg/kg oral dexamethasone ) had a significant improvement in both retraction from 2.0 ( range 1-3) to 0.5 (range 0-3); p<0.002) and in stridor from 2 (range 0- 2 ) to 0.5 (range 0- 1); p<0.001). Group B (0.15 mg/kg, oral dexamethasone) showed, also, significant improvement in both re-traction and stridor. However, there was no statistical significant difference between the two groups regarding retraction and stridor score after the 24 hours with p value =0.87.
No statistical significant difference was evident between the two groups at 12 and 24 hours after treatment neither in oxygen saturation nor in the respiratory rate. The rate of hospital admission (Table 3) after treatment in group A (0.6mg/kg dexa-methasone) was 15/36 children (41.6 percent) and it was 14/36 children (38.9 percent) in group B (0.15mg/kg dexamethasone). The value were not statistically significant (p value = 0.36)
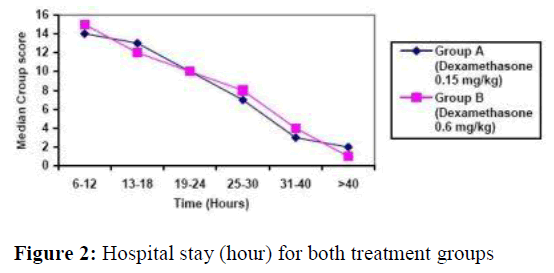
The median hospital stay (hours) of group A (0.6mg/kg dexamethasone) was 28 (12-50) and in group B (0.15 mg/kg dexamethasone) was 26 (14- 48). There was no statistical significant difference between the two groups (t=-0.32, p=0.64) (figure 2). Four patients from each group were admitted into pediatric ICU but none of them needed mechanical ventilation.
Two patients developed bronchopneumonia on the second day of admission as confirmed by chest X-ray and one patient had bacterial trachitis. All these three patients were in group A (0.6 mg/kg dexamethasone). No adverse events were noted in the group B patients. No patient had clinical deterioration, either in the emergency room or after discharge and no child had gastrointestinal bleeding or bacterial infection [26].
No difference was seen between the two groups with regard to the use of nebulized adrenaline either in first hour post-treatment or subsequently (Table 3). There was no difference in the pulse rate, respiratory rate, temperature, or oxygen saturation (in room air) between the two groups at presentation or at the time of discharged from the emergency room or during hospitalization.
Although no attempt was made to categorize children‟s illness as viral or episodic croup, on entry into the trail, we recorded whether children had symptoms of fever and rhinorrhea at home or/and had fever in hospital. Also, the number of previous episodes of croup, the number of admissions to hospital with croup, and the duration of symptoms prior to presentation, were recorded. We arbitrarily defined viral croup as having temperature of greater than 38°C/or fever and rhinorrhea at home and symptoms for 6 hr or more. The episodic spasmodic group was defined as no history of fever and rhinorrhnea at home, fever duration of less than 4 hrs, and at least one episode of croup in the past. Based on these definitions we were able to label the patient‟s illness as either viral or spasmodic. There was no dif-ference in the proportion of viral, spasmodic, or undefined children‟s illness in either group. There was no significant difference in the duration of hospitalization between the viral- and the spasmodic- causes of children‟s illness.
Discussion
There has been increasing evidence of an immunological component for the acute laryngotracheobronchitis. Welliver et al [27] reported that children with croup caused by parainflunza viruses had high titers of both parainflunza virus-specific IGE and histamine in their nasal secretion in comparison with children who had only an upper respiratory tract infection caused by parainflunza virus. Furthermore, over half of the children with past history of croup developed increased airway hyperactivity and have abnormal pulmonary function [28,29]. The use of dexamethasone in acute viral croup may have a role in blunting these immunological processes and hence potentially ameliorating not only the acute process but also these later responses. Further studies are needed to confirm this hypothesis.
In our randomized, double-blind, controlled trials involving children with croup who presented to the emergency room, we used two different doses of dexamethasone, 0.6mg/kg in one group and a smaller dose of 0.15 mg/kg in the other group. The croup score was used as primary outcome variable in our study because it represents the severity of the patient‟s illness and could easily be applied in clinical practice [5,24]. The improvement of total clinical score at 12 hours and 24 hours in patients received small dose of oral dexamethasone (0.15mg/kg) was similar to the clinical improvement in patient received the stan-dard dose of oral dexamethasone (0.6mg/kg).
Stridor and retractions which are the major components of the croup score, correlate well with the diameter of the trachea when it is measured fluoroscopically [30]. Most of the improve-ment in the total croup score in our trial was seen in stridor and retraction parameters. Both treatment groups showed significant improvement at different intervals of the study, and there was no statistical difference between the two groups (p value <0.05).
In this study, the rate of hospitalization in group A (0.6 mg/kg oral dexamethasone) was 41.6 percent and in group B (0.15 mg/kg oral dexamethasone) 38.9 percent and there was no significant difference noted between the two groups with odd ratio for differences between treatments (95% CI), 0.4 (0.2-1.3). Hospital admission rate for children suffering from croup which had been seen in outpatient setting ranged from 1.5% to 30% of cases seen. These figures vary widely, depending on hospital admission practices and the severity of the disease in the popu-lation being assessed [31,32]. Admission rate in patients at-tending emergency room was higher and it has been shown by other investigators that hospital admission rate is reduced in patient treated with 0.6 mg/kg of dexamethasone. Johnson et al [33] demonstrated that the rate of hospitalization after treatment in the placebo group was 67 percent and it was reduced to 35 percent in patients treated with steroids.
Our study also showed that treatment with small dose of oral dexamethasone (0.15mg/kg) resulted in no significant difference in the duration of hospitalization as compared to the standard dose of oral dexamethasone (0.6mg/kg). Hospital stay has been shown to be shorter in patients treated with dexamethasone as compared to placebo. Geelhood and Macdonald, [21] demon-strated that patients treated with steroids have shorter duration of hospitalization as compared to placebo group.
We found that there is a little need for adrenaline administration after the first two hours post-treatment in patients who received small dose of oral dexamethasone (0.15mg/kg), as it is the case in patients treated with standard dose of oral dexamethasone (0.6mg/kg). This decrease in the use of adrenaline was also found by Super et al [17].
Our data did not demonstrate any significant differences in respiratory rate, oxygen saturation or intensive care unit admis-sions between the two groups.
In the meta-analysis study, there appeared to be a dose-response effect of steroids in croup. The great effect clinical improvement was seen in studies in which the highest doses of steroids were used [9,10,15]. The low doses studies in the meta-analysis, however, used an average dose of only 0.08mg/kg of dexamethasone [11,12,14]. These findings are compatible with the existence of a plateau effect where an improvement in the outcome occurs with increasing doses up to somewhere between 0.08 and 0.15 mg/kg dexamethasone with no further improvement achieve with greater doses and this is also demonstrated in our data analysis as above. In addition to our study, Geelhood et al [34] also concluded that oral dexamethasone in a dose of 0.15 mg/kg is effective in out-patient children with acute laryngotracheobronchitis.
Regarding the limitation of our study, there was no placebo group included in our trial. However, since the benefit of steroids in the treatment of laryngotracheobronchitis have been established [20,21,35-36], we think that it is unethical to deprive patients from this modality of treatment. In addition, there was no long-term follow-up of patients and the type of causative organism was not isolated and the clear distinction between viral laryngotracheobronchitis and spasmodic croup was made only by clinical assessment. However, a traditional distinction has been made between spasmodic recurrent croup and laryngotracheobronchitis. Some researchers have considered the distinction is important because spasmodic croup may have an allergic component [37] and may improve more rapidly than laryngotracheobronchitis whether or not treatment is given, whereas laryngotracheobronchitis is thought to be clearly associated with viral infection of the respiratory tract, especially, but not exclusively, infection with parainflunza virus [38]. Others recognized that there is enough overlapping in the signs, symptoms and viral origins of the spasmodic croup and laryngotracheobronchi-tis to consider them a manifestation of a single disease [3]. Johnson et al [33] did not find that a viral-induced croup had a significant effect on the difference between the treatment groups. The efficacy of glucocorticoids has been demonstrated in children with both types of croup [21].
Regarding adverse effects in our entire study population, three patients in group A (0.6mg/kg dexamethasone) developed com-plications. Two patients developed pneumonia and one patient developed bacterial trachitis. None of the patients in group B (0.15mg/kg) developed any adverse effects. These observed complication have been reported previously in patients with croup who were treated with dexamethasone. In Jame‟s study [8] eight out of 88 patients (9%) developed pneumonia, of whom 2 of 8 (25%) received dexamethasone. In the study by Muhlen-hal et al [10] bronchopneumonia or bronchitis developed in 24 of 349 (7%) patients; 18% of the 24 patients received dexamethasone. One explanation for the development of pneumonia and bacterial trachitis in our trail is that these complications are related to the natural course of the illness. However, the possibility that these complications could be due to dexamethasone cannot be ignored and the risk of pneumonia and bacterial trachitis must be balanced against the benefits of using dexa-methasone in the treatment of children with laryngotracheobronchitis. Further studies are needed to evaluate the safety of this treatment in such viral illnesses.
Past advocation against the routine use of glucocorticoids in patients with croup have stressed the potential for infrequent serious gastrointestinal hemorrhage and other adverse effects after dexamethasone therapy [25,26]. Glucocorticoids should be given with caution in patients with preexisting immunodeficiency, recent exposure to varicella, or possible tuberculosis. When used in previously healthy children with croup, however, glucocorticoids have had a few important adverse effects [3,35].
In conclusion, our trial demonstrated that oral dexamethasone at a dose of 0.15 mg/kg is as effective as 0.6mg/kg in reducing the total croup score, the need for racemic epinephrine, the rate of hospital admission and the duration of hospitalization. There were no statistical differences in respiratory rate, oxygen satura-tion or intensive care admission. Two patients had pneumonia and one had bacterial trachitis and these patients were treated with 0.6 mg/kg dexamethasone. No adverse effects were seen in patients treated with the small dose of dexamethasone (0.15mg/kg). Therefore, we recommend further studies to ex-plore the safety of glucocorticoids and the efficacy at different doses of dexamethasone in treating children with acute laryngotracheobronchitis. In addition, we advice that specimens for viral studies are to be obtained by a nasal swab (for respiratory syncytial virus antigen assay by fluorescent antibody [39], a throat swab (for culture of parainflunza and influenza virus) and other methods to be examined by standard laboratory procedures [40].
Acknowledgements
The authors gratefully acknowledge the invaluable contribution of the nurses and the medical staffs at Aseer Central Hospital , Abha Private Hospital and Medical Consultative Center , Abha City, Southwestern Saudi Arabia. Likewise, the helpful criticism of the manuscript by Professor Olu Ozinno. The parents and their children who participated in this trial of research are also appreciated. Also thanks to Mr. Allan Agaton for his secretarial assistance in the preparation of this manuscript.
References
- Denny FW, Murphy TF, Clyde WA Jnr, et al Croup: an 11-year study in a pediatric practice. Pediatrics 1983; 71: 871-876.
- Skolnik NS. Treatment of croup: a critical review. Am J Dis Child 1989; 143: 1045-1049.
- Waisman Y, Klein BL, Boenning DA, et al Prospective ran-domised double-blind study comparing L-epinephrine and racemic epinephrine aerosols in the treatment of laryngotra-cheitis (croup). Pediatrics 1992; 89: 302-306.
- Epinephrine aerosols in the treatment of laryngotracheitis (croup). Pediatrics 1992; 89: 302-306.
- Westley CR, Cotton EK, Brooks JG. Nebulized racemic epi-nephrine by IPPB for the treatment of croup: a double-blind study. Am J Dis Child 1978; 132: 484-487.
- Novik, A. Corticosteroid treatment of non-diphtheritic croup. Acta Otolaryngol 1960;158: 2023.
- Martensson, B., Nilson, G., & Torbjar, J. The effect of corti-costeroids in the treatment of pseudo-croup. Acta Otolaryngol 1960; 158: 6271.
- James JA. Dexamethasone in croup: a controlled study. Am J Dis Child 1969; 117: 511-516.
- Leipzig B, Oski FA, Cummings CW, Stockman JA, Swender P. A prospective randomized study to determine the efficacy of steroids in the treatment of croup. J Pediatr 1979; 94: 194-196.
- Muhlendahl KE v, Kahn D, Spohr HL, Dressler F. Steroid treatment in pseudocroup. Helvetica Paediatrica Acta 1982; 37: 431-436.
- Sussman S, Grossman M, Magoffin R, Schieble J. Dexa-methasone (16 alpha- methyl, 9 alphafluoroprednisolone) in obstructive respiratory tract infections in children: a controlled study. Pediatrics 1964; 34: 851-855.
- Eden AN, Larkin VP. Corticosteroid treatment of croup. Pedi-atrics 1964; 33: 768-769.
- Skowron PN, Turner JA, McNaughton GA. The use of corticosteroid (dexamethasone) in the treatment of acute laryngotracheitis. Can Med Assoc J 1966; 94: 528-53.
- Eden AN, Kaufman A, Yu R. Corticosteroids and croup: controlled double-blind study. JAMA 1967; 200: 403-404.
- Koren G, Frand M, Barzilay Z, MacLeod SM. Corticosteroid treatment of laryngotracheitis vs. spasmodic croup in children. Am J Dis Child 1983; 137: 941-944.
- Tunnessen WW, Feinstein AR. The steroid-croup contro-versy: an analytical review of methodological problems. J Pediatr 1980; 96: 751-756.
- Super DM, Cartelli NA, Brooks U, Lembo RM, Kumar ML. A prospective randomized double-blind study to evaluate the effect of dexamethasone in acute laryngotracheitis. J Pediatr 1989; 115: 323-329.
- Kuuseia AL, Vesikari T. A randomized double-blind, placebo-controlled trial of dexamethasone and racemic epinephrine in the treatment of croup. Acta Paediatr Scand 1988; 77:99-1 04.
- Tibballs J, Shann FA, Landau LI. Placebo-controlled trial of prednisolone in children intubated for croup. Lancet 1992; 340: 745-748.
- Husby 5, Agertoft L, Mortensen 5, Pedersen S. Treatment of croup with nebulised steroid (budesonide): a double blind, placebo controlled study. Arch Dis Child 1993; 68: 352-355.
- Geeihoed GC, Macdonald WB. Oral and inhaled steroids in croup: a randomized, placebo-controlled trial. Pediatr Pulmonol 1995; 20: 355-361.
- Kastrup EK, Olan B III, eds. Drug facts and comparisons. St. Louis: JB Lippincott, 1988: 346.
- Yates RW, Doull IJ. A risk-benefit assessment of corticosteroids in the management of croup. Drug Saf 1997; 16: 48-55.
- Johnson DW, Schch 5, Koren G, Jaffe DM. Outpatient treat-ment of croup with nebulized „„dexamethasone. Arch Pediatr Adolesc Med l996; 150: 349-355.
- Brown GW. Counts, scales, scores: Levels of observation. Am J Dis Child 1985; 139:147-151.
- Smith DS. Corticosteroids in croup: a chink in the ivory tower? J Pediatr 1989; 115: 256-257.
- Welliver RC, Wong DT, Middeton E, Sun M, McCarthy N, Ogra PL. Role of parainflunza virus-specific IgE in pathogensis of croup and wheezing subsequently to infection. J Pedi-atr 1982; 101: 889-896.
- Loughlin GM, Taussig LM. Pulmonary function in children with a history of laryngotracheobronchitis. J Pediatr 1979; 94: 365-369.
- Zach M, Erben A, Olinsky A. Croup, recurrent croup, allergy and airways hyperreactivity. Arch Dis Child 1981; 56: 336-341
- Corkey CWB, Barker GA, Edmonds JF, Mok PM, Newth CJL. Radiographic tracheal diameter measurements in acute infectious croup: An objective scoring system. Crit Care Med 1981; 9: 587-590.
- To, T. & Young, W. Hospitalizations for croup in Ontario [ab-stract]. Clin Invest Med 1994; 17: A25.
- Marx, A, Torok, TJ. Holman, RC., & Clarke, MJ. Pediatric hospitalizations for croup (laryngotracheitis): biennal increases associated with human parainfluenza virus 1 epide-mics. J Infect Dis 1997; 176: 1423-1427.
- Johnson DW, Jacobson 5, Edney PC, Hadfield P, Mundy ME, Schuh S. A comparison of nebulized budesonide, intramuscular dexamethasone, and placebo for moderately severe croup. N Engl J Med 1998; 339: 498-503.
- Geelhoed GC, Turner J, Macdonald WBG. Efficacy of a small single dose of oral dexamethasone for outpatient‟s croup: a double blind placebo controlled clinical trial. BMJ 1996; 313: 140-142.
- Kairys SW, Olmstead EM, O‟Connor GT. Steroid treatment of laryngotracheitis: a meta-analysis of the evidence from ran-domized trials. Pediatrics l989; 83: 683- 693.
- Klassen TP, Feldman ME, Watters LK, Sutcliffe T, Rowe PC. Nebulized budesonide for children with mild to moderate croup. N Engl J Med 1994; 331: 285-289. [Abstract/Free Full Text]
- Zach MS, Schnall RP. Landau LI. Upper and lower airway hyperreactivity in recurrent croup. Am Rev Respir Dis 1980; 121: 979-983.
- Henrickson KJ, Kuhn SM. Savatski LU. Epidemiology and cost of infection with human parainfluenza virus types 1 and 2 in young children. Clin Infect Dis 1997; 18: 770-779.
- Kumar M, Super D, Lembo R, Thomas F, Prokay S. Efficacies of two rapid respiratory syncytial virus diagnostic tests. J Clin Microbial 1987; 25: 873-875.
- Lennette EH. Diagnostic procedures for viral, rickettsial and chlamydial infections. Washington D.C.: American Public Health Association, 1979.