Research Article - Journal of Cardiovascular Medicine and Therapeutics (2023) Volume 7, Issue 5
Efficacy and safety of sacubitril/valsartan in left ventricular assist devices: A single center experience.
Abdulrahman Nazif1*, Maya Guglin2, Naveed Akhtar3, Laurie Schenkelberg4, Kutaiba Nazif5, Onyedika J Ilonze2
1Department of Medicine, Indiana University, Indianapolis, Indiana, United States
2Department of Cardiovascular Medicine, Indiana University, Indianapolis, Indiana, United States
3Department of Cardiology, Advanced Heart Failure Center, Piedmont Augusta, GA, United States
4Department of Pharmacy, Indiana University, Indianapolis, Indiana, United States
5Department of Cardiology, Lehigh Valley Heart and Vascular Institute, Allentown, PA, United States
- Corresponding Author:
- Abdulrahman Nazif
Department of Medicine,
Indiana University,
Indianapolis,
Indiana,
United States
E-mail: anazif@iu.edu
Received: 03-Apr-2023, Manuscript No. AACMT-23-93912; Editor assigned: 05-Apr-2023, AACMT-23-93912 (PQ); Reviewed: 19-Apr-2023, QC No. AACMT-23-93912; Revised: 23-Jun-2023, Manuscript No. AACMT-23-93912 (R); Published: 30-Jun-2023, DOI:10.35841/aacmt.7.5.161
Citation: Nazif A, Guglin M, Akhtar N, et al. Efficacy and safety of sacubitril/valsartan in left ventricular assist devices: A single center experience. J Cardiovasc Med Ther. 2023;7(5):1-5.
Keywords
Ventricular assist device, Heart failure, Sacubitril-valsartan, Guideline directed medical therapy, Angiotensin receptor neprilysin inhibitor
Introduction
Guidelines Directed Medical Therapy (GDMT) for HF includes the angiotensin receptor neprilysin inhibitor Sacubitril/ Valsartan (S/V) [1,2]. The use of GDMT in patients with advanced Heart Failure (HF) on Left Ventricular Assist Devices (LVADs) is increasing. A retrospective analysis from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) on the effect of neurohormonal antagonism of all GDMT apart from Sacubitril/Valsartan (S/V) on LVAD patients showed improved survival and quality of life [3]. Furthermore, retrospective data suggests a possible mortality benefit and reduced risk of major gastrointestinal bleeding with neurohormonal antagonism post-LVAD [4,5]. Although prior trials showed benefit of S/V therapy over reninangiotensin- aldosterone antagonists in ambulatory HF, the LCZ696 in advanced heart failure (LIFE) trial showed poor tolerability of S/V and similar outcomes when compared to Angiotensin Receptor Blockers (ARBs) in an advanced HF patient population [6,7].
Thus, neurohormonal modulation in advanced HF may be alleviated but not abolished by LVAD therapy, suggesting a possible benefit from HF GDMT post-LVAD implantation. Prior studies on S/V use in patients on LVAD support have been limited by small sample size, inadequate power and short (<6 months) follow up duration [8-11]. To overcome this knowledge gap, we conducted a retrospective single center analysis of patients with LVAD who tolerated S/V at our center from 2017-2021.
Materials and Methods
We retrospectively reviewed patients implanted with LVAD support at Indiana university methodist hospital between January 2017 and December 2021 who tolerated S/V therapy for at least 1 month. Baseline characteristics obtained included age, sex, Body Mass Index (BMI), coronary artery disease, diabetes status and baseline kidney function. Data on S/V dosing and HF drug therapy was also gathered. We reported changes in Mean Arterial blood Pressure (MAP), serum creatinine, serum potassium and NT-proBNP. In our institution, MAP is routinely monitored by both Doppler ultrasound and BP cuff. Trends in LVAD parameters including power, speed and flow at baseline clinic visit and at 3, 6 and 9 months follow up visits or within a week of hospitalization were also obtained. Intolerability to S/V was defined as: Acute kidney injury-a rise in serum creatinine >0.3 mg/dL from baseline, hyperkalemia (>5.5 mmol/L), dizziness or hypotension.
We did an additional analysis based on renal function of the patients at baseline. Because of the small sample size, we divided the subjects into two equal parts based on the baseline serum creatinine. We then compared the variables in between the groups with lower and higher serum creatinine. Continuous variables were evaluated for distribution normality using the Shapiro-Wilk test and were expressed as mean ± standard deviation or median and Interquartile Range (IQR) and compared using independent sample t-test for normally distributed variables or Mann-Whitney test for non-normally distributed variables. Categorical variables were compared using the chi-square test. All statistical significance was assessed using a 2 sided P values, with p-value <0.05 considered statistically significant. Data were analyzed using IBM SPSS 21.0 statistical software (IBM SPSS version 21.0. Armonk, NY).
Results
A total of 24 patients were started on S/V in our program and met the inclusion criteria. The mean age of patients in our cohort is 50 years old (IQR 40-63) with 19/79% being male. Mean Body Mass Index (BMI) was 37.5 kg/m2. Common comorbidities observed in our cohort were coronary artery disease in 12/50% and diabetes mellitus in 14/58% (Table 1). A third, 8/33.3% patients had chronic kidney disease with an estimated Glomerular Filtration Rate (eGFR) <60 mg/dL. The most frequently implanted LVAD type was the heartmate 3, (Abbott, Abbott Park, IL) 16/66.7%, followed by Heartmate II (HM II) 4/16.6% and HeartWare (Medtronic, Minneapolis, MN) 4/16.6%. Most of the patients 19/79% had LVAD implanted as destination therapy and 5/20.8% as bridge to transplant.
| Demographics | N=24 |
|---|---|
| Age (years) | 50 (IQR 40-63) |
| Male, n (%) | 19 (79%) |
| BMI (kg/m2) | 37.5 (SD 9.5) |
| Mean arterial pressure (mmHg) | 83 (SD 15.4) |
| Comorbidities, n (%) | |
| Chronic kidney disease | 8 (33%) |
| Diabetes mellitus | 14 (58%) |
| Coronary artery disease | 12 (50%) |
| Heart failure medications, n (%) | |
| ACEI/ARB or S/V pre LVAD implantation | 13 (54%) |
| Beta blockers | 20 (83%) |
| Mineralocorticoid receptor antagonist | 17 (71%) |
| Sodium glucose cotransporter-2 inhibitors | 2 (8%) |
| Phosphodiesterase inhibitors | 2 (8%) |
| Digoxin | 6 (25%) |
| S/V dose (mg), n (%) | |
| 24/26 | 16 (66.6%) |
| 49/51 | 6 (25%) |
| 97/103 | 2 (8.3%) |
| Number of days from LVAD implantation to S/V initiation | 308 (IQR 11-642) |
| Reasons for intolerance of S/V, n (%) | |
| Postural dizziness or symptomatic hypotension | 2 (8%) |
| Rise in serum creatinine (>0.3 mg/dl) | 2 (8%) |
| Hyperkalemia | 0 (0%) |
| Type of LVAD, n (%) | |
| HeartMate 3 | 16 (66.6%) |
| HeartMate II | 4 (16.6%) |
| Medtronic HeartWare | 4 (16.6%) |
| LVAD parameters | |
| Power (watts) | 4.6 (SD 1.19) |
| Flow (liters/minute) | 4.7 (SD 1.3) |
| Speed (rpm) | |
| HeartMate 3 (speed range 5,000 to 6,000) | 5294 (SD 303.5) |
| HeartMate II (speed range 8,800 to 10,000) | 7354.1 (SD 416.3) |
| Medtronic HeartWare (speed range 2,400 to 3,200) | 2148.5 (SD 222.4) |
Note: IQR: Interquartile Range, SD: Standard Deviation, BMI: Body Mass Index, eGFR: estimated Glomerular Filtration Rate, ACEI: Angiotensin Converting Enzyme Inhibitors, ARB: Angiotensin Receptor Blockers, S/V: Sacubitril/Valsartan, LVAD; Left Ventricular Assist Device.
Table 1. Baseline characteristics.
In terms of HF GDMT at the time of S/V initiation, 20/83% was taking a beta blocker, 17/71% was taking a mineralocorticoid receptor antagonist, 23/96% was taking a loop diuretic, while 2/8% was on phosphodiesterase inhibitor. In terms of renin-angiotensin-aldosterone neurohormonal antagonism, 4/16.6% were on Angiotensin Converting Enzyme Inhibitors (ACEIs), 2/8% on Angiotensin Receptor Blockers (ARBs) and 7/29% had a history of being on S/V prior to LVAD implantation. Of those who tolerated S/V, 14/74% were on low dose, 4/21% were on medium dose, and 1/5% on high dose. Patients who tolerated S/V were started on S/V at a median of 308 days (IQR 11-642) from the time of LVAD implant. The discontinuation rate of S/V over a 9 months follow up was 4/17%. Of these patients, two patients discontinued S/V therapy due to dizziness and orthostatic hypotension, while two other patients discontinued due to the development of acute kidney injury. Additionally, one patient was lost to follow up.
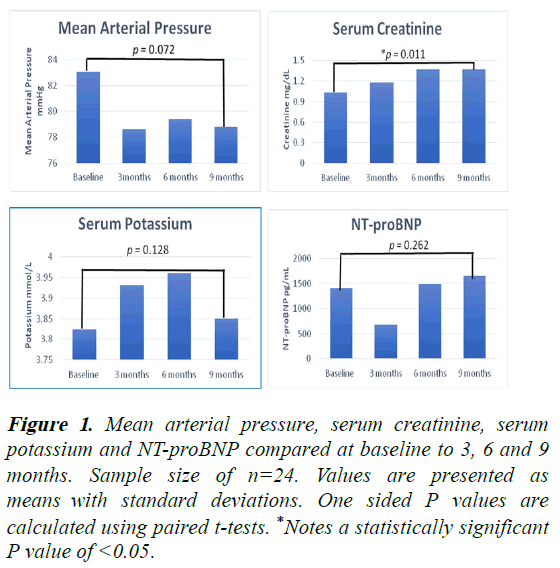
At the time of S/V initiation, the MAP was 83 mmHg (SD 15.4) and the MAP was 78.6 mmHg (SD 12.6) at 3 months, 79.4 mmHg (SD 12.7) at 6 months and 78.8 mmHg (SD 13.4) at 9 months, (p=0.072) (Figure 1). There was a significant increase in serum creatinine. The corresponding values for serum creatinine were 1.03 mg/dL (SD 0.32) at S/V initiation, 1.18 mg/dL (SD 0.3) at 3 months, 1.37 mg/dL (SD 0.47) at 6 months and 1.37 mg/dL (SD 0.59) at 9 months, (p=0.011). A rise in serum creatinine (>0.3 mg/dl) was noted in 11/46%, but this only led to total discontinuation of S/V in 2 patients. The mean serum potassium on initiation of S/V was 3.8 mmol/L (SD 0.4), 3.9 mmol/L (SD 0.4) at 3 months, 4 mmol/L (SD 0.37) at 6 months and 3.9 mmHg (SD 0.42) at 9 months, (p=0.128). In terms of natriuretic peptides, the mean NTproBNP levels at baseline (initiation of S/V) was 1406 pg/ml (SD 1338), 678 pg/ml (SD 452) at 3 months, 1486 pg/ml (SD 2386) at 6 months and 1650 pg/ml (SD 1936) at 9 months, (p=0.262). The average baseline flow is 4.7 lpm (SD 1.3), 3 months mean flow of 4.77 lpm (SD 1), 6 months mean flow of 4.87 lpm (SD 1.1) and 9 months mean flow of 4.67 lpm (SD 1.2). This change was not statistically significant, (p=0.449) (Table 2).
Figure 1: Mean arterial pressure, serum creatinine, serum potassium and NT-proBNP compared at baseline to 3, 6 and 9 months. Sample size of n=24. Values are presented as means with standard deviations. One sided P values are calculated using paired t-tests. *Notes a statistically significant P value of <0.05.
| Measurement | Baseline | 3 months | 6 months | 9 months | P-value |
|---|---|---|---|---|---|
| Mean arterial pressure (mmHg) | 83 (15.4) | 78.6 (12.6) | 79.4 (12.7) | 78.8 (13.4) | 0.072 |
| Creatinine (mg/dL) | 1.03 (0.32) | 1.18 (0.3) | 1.37 (0.47) | 1.37 (0.59) | 0.011* |
| Potassium (mmol/L) | 3.8 (0.4) | 3.9 (0.4) | 4 (0.37) | 3.9 (0.42) | 0.128 |
| NT-proBNP (pg/mL) | 1406 (1338) | 678 (452) | 1486 (2386) | 1650 (1936) | 0.262 |
| LVAD parameters | |||||
| Power (watts) | 4.55 (1.18) | 4.5 (1.1) | 4.6 (1.1) | 4.6 (1.2) | 0.826 |
| Flow (liters/minute) | 4.7 (1.3) | 4.7 (1) | 4.8 (1.1) | 4.6 (1.2) | 0.449 |
| Speed | |||||
| HeartMate 3 (5,000-6,000) | 5294 (303) | 5275 (355) | 5330 (316) | 5636 (367) | 0.472 |
| HeartMate II (8,800-10,000) | 7354 (416) | 7354 (416) | 7415 (462) | 7415 (462) | 0.211 |
| HeartWare (2,400-3,200) | 2148 (222) | 2159 (233) | 2149 (267) | 2149 (267) | 0.384 |
Note: Data is presented as mean (standard deviation). *Indicates significant p-value of <0.05. All p-values were calculated using IBM SPSS 21.0 statistical software.
Table 2. Clinical, laboratory and LVAD parameters pre-sacubitril/valsartan initiation to the 3, 6 and 9 month follow up periods.
We performed an additional analysis based on the renal function of the patients at the baseline. In the equal cohorts, in subjects with lower serum creatinine, the levels were at 0.8 ± 0.15 mg/dL, 1.13 ± 0.35 mg/dL, 1.18 ± 0.30 mg/dL and 1.2 ± 0.39 mg/dL at the baseline, 3 months, 6 months, and 9 months, respectively, with a significant interval increase (p=0.009). In patients with higher serum creatinine, the values were 1.28 ± 0.25 mg/dL, 1.35 ± 0.26 mg/dL, 1.53 ± 0.54 mg/dL and 1.59 ± 0.69 mg/dL at the baseline, 3 months, 6 months and 9 months, respectively, with no significant interval increase (p=0.18). We then compared the variables in between the groups with lower and higher serum creatinine. The MAP decreased over the study period in the high creatinine group from 84.8 ± 12.2 mmHg at the baseline to 72.4 ± 10.1 mmHg at 9 months (p=0.0018) but not in the low creatinine group (87 ± 19.5 mmHg at baseline to 88 ± 10.3 mmHg at 9 months, p=0.54). Other parameters, including heart rate, serum potassium, NTproBNP and pump parameters, did not differ depending on renal function.
Discussion
Our retrospective analysis represents one of the largest studies with the longest follow-up on tolerability and effects of sacubitril/valsartan in patient supported by LVADs. The only larger dataset was reported by investigators from Columbia university (30 patients), but they had a shorter follow-up and did not provide detailed data on LVAD pump parameters [12]. We demonstrated an overall good tolerability of these drugs. We also showed, for the first time, a statistically significant increase in serum creatinine, while no change in blood pressure, serum potassium or NT-proBNP levels. Unlike other studies, we did not see a significant reduction in NT-proBNP or MAP and saw a significant rise in serum creatinine. When we dichotomized patients into lower and higher creatinine, we saw a significant decrease in MAP only in patients with higher serum creatinine (84.8 ± 12.2 mmHg to 72.4 ± 10.1 mmHg, p=0.0018). There was no statistical difference in the LVAD flow. Other studies such as Sharma, et al. showed a decrease in MAP without acute kidney injury or hyperkalemia in 5 LVAD patients while Alishetti, et al. showed a decrease in MAP and NT-proBNP in 40 LVAD patients on S/V.
The significant rise in creatinine in our cohort did not meet criteria for acute kidney injury and led to discontinuation of S/V in only two patients. However, without a control group, it is unclear whether S/V alone is responsible for the serum creatinine rise. Although kidney disease is common in patients with advanced HF, renal function may improve in the short term period following LVAD implantation especially if there was pre-existing cardiorenal syndrome [13]. However over the long term, there may be a decline in renal function. Multiple studies which have characterized renal function with S/V use in patients supported with LVAD have been retrospective, of short duration (<6 months) and had small sample sizes. Furthermore, the rise in creatinine in our cohort may be reflective of the diuretic/natriuretic effect of S/V due to the neutral endopeptidase inhibition by sacubitril leading to the enhanced activity of endogenous natriuretic peptides [14].
Although a trend towards a decrease in MAP was noted in our study, it was not statistically significant and the MAP remained well within therapeutic range throughout the follow-up period. In patients with lower renal function (higher serum creatinine), the MAP decreased throughout the study and was significantly lower at the end of the 9 months follow-up period than at baseline, but still remained within normal range. Optimal blood pressure control in LVAD patients is beneficial as it leads to greater pump output, reduced risk of stroke, aortic regurgitation and pump thrombosis [15]. However, these benefits should be juxtaposed with LVAD patients who may have orthostatic hypotension with neurohormonal antagonism.
Thus, S/V may be beneficial in carefully selected LVAD patients, especially hypertensive LVAD patients. The increase in serum potassium we observed was not significant and hyperkalemia was not seen and no patient discontinued S/V due to hyperkalemia. Initiation of S/V showed no significant difference in NT-proBNP levels at 9 months (p=0.262). Some retrospective single center studies have also shown no hyperkalemia and reduction in NT-proBNP with S/V with LVADs. The lack of change to slight increase in NT-proBNP levels in contrast to the reduction seen in other retrospective studies may be due to the longer follow up of patients in our cohort. It may also be that NT-proBNP levels have an initial drop but over a longer period of follow up the NT-proBNP are not different from baseline. We also found tolerability of S/V to parallel the major S/V LIFE trial with discontinuation rate of 17% compared to 18% in the LIFE trial. Our study adds to the growing evidence of the safety and tolerability of S/V in patients with durable LVAD support.
This study has limitations. It is a single center and retrospective analysis with a small sample size hence may be underpowered to detect a significant difference. However, our paper represents the longest patient follow up of 9 months for LVAD patients on S/V when compared to recent studies (<3 months).
Conclusion
In our cohort of patients on LVAD support, S/V was overall well tolerated with a drop-out rate due to intolerance (hypotension, renal dysfunction) of 17%. The patients, who stayed on S/V for at least 9 months, had a significant increase in serum creatinine. In the whole cohort, no significant changes in blood pressure, serum potassium or NT-proBNP occurred. However, in patients with higher baseline creatinine, MAP decreased significantly, but remained within normal range. It appears that S/V is overall safe in this patient population. Prospective controlled studies are needed to establish the efficacy of S/V in patients on LVAD support and to compare them with the existent options of ACE or ARBs.
References
- Velazquez EJ, Morrow DA, de Vore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Eng J Med. 2019;380(6):539-48.
[Crossref] [Google Scholar] [PubMed]
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Eng J Med. 2014;371(11):993-1004.
[Crossref] [Google Scholar] [PubMed]
- McCullough M, Caraballo C, Ravindra NG, et al. Neurohormonal blockade and clinical outcomes in patients with heart failure supported by left ventricular assist devices. JAMA Cardiol. 2020;5(2):175-82.
[Crossref] [Google Scholar] [PubMed]
- Yousefzai R, Brambatti M, Tran HA, et al. Benefits of neurohormonal therapy in patients with continuous flow left ventricular assist devices. ASAIO J. 2020;66(4):409-14.
[Crossref] [Google Scholar] [PubMed]
- Converse MP, Sobhanian M, Taber DJ, et al. Effect of angiotensin II inhibitors on gastrointestinal bleeding in patients with left ventricular assist devices. J Am Coll Cardiol. 2019;73(14):1769-78.
[Crossref] [Google Scholar] [PubMed]
- Vader JM, Givertz MM, Starling RC, et al. Tolerability of sacubitril/valsartan in patients with advanced heart failure: Analysis of the life trial run-in. JACC Heart Fail. 2022;10(7):449-56.
[Crossref] [Google Scholar] [PubMed]
- Mann DL, Givertz MM, Vader JM, et al. Effect of treatment with sacubitril/valsartan in patients with advanced heart failure and reduced ejection fraction: A randomized clinical trial. JAMA Cardiol. 2022;7(1):17-25.
[Crossref] [Google Scholar] [PubMed]
- Alishetti S, Braghieri L, Jennings DL, et al. Angiotensin receptor neprilysin inhibitor use in patients with left ventricular assist devices: A single center experience. Int J Artif Organs. 2022;45(1):118-20.
[Crossref] [Google Scholar] [PubMed]
- Randhawa VK, West L, Luthman J, et al. Sacubitril/valsartan in patients post-left ventricular assist device implant: A single centre case series. Eur J Heart Fail. 2020;22(8):1490-2.
[Crossref] [Google Scholar] [PubMed]
- Sharma A, Moayedi Y, Duclos S, et al. Tolerability of sacubitril/valsartan in patients with durable left ventricular assist devices. ASAIO J. 2020;66(3):e44-5.
[Crossref] [Google Scholar] [PubMed]
- Zorz N, Frljak S, Vrtovec B. Effects of sacubitril/valsartan in patients with left ventricular assist devices: Case series. Artif Organs. 2021;45(2):185-6.
[Crossref] [Google Scholar] [PubMed]
- Dobarro D, Diez-Lopez C, Couto-Mallon D, et al. Use of sacubitril-valsartan in blood pressure control with left ventricular assist devices. J Heart Lung Transplant. 2020;39(12):1499-501.
[Crossref] [Google Scholar] [PubMed]
- Roehm B, Vest AR, Weiner DE. Left ventricular assist devices, kidney disease and dialysis. Am J Kidney Dis. 2018;71(2):257-66.
[Crossref] [Google Scholar] [PubMed]
- Vardeny O, Claggett B, Kachadourian J, et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: The PARADIGM-HF trial. Eur J Heart Fail. 2019;21(3):337-41.
[Crossref] [Google Scholar] [PubMed]
- Briasoulis A, Ruiz Duque E, Mouselimis D, et al. The role of renin-angiotensin system in patients with left ventricular assist devices. J Renin Angiotensin Aldosterone Syst. 2020;21(4):1-10.
[Crossref] [Google Scholar] [PubMed]