Keywords
|
| Combined etanercept and iguratimod, Rheumatoid, Arthritis, Clinical trial |
Introduction
|
| Subjects treated with etanercept plus MTX have improvement compared with those treated with ETN alone [1]. The studies have shown that etanercept (trade name: Enbrel) quickly takes effect when treating moderate to severe active rheumatoid arthritis, which is more effective than MTX and has good safety; meanwhile, after 52 weeks of treatment, subjects treated with etanercept plus MTX have significantly improved joint Sharp score compared with those treated with MTX alone; moreover, radiological findings suggest that the combination therapy can effectively prevent bone and joint damage [2-4]. Meanwhile, efficacy and safety comparison studies between iguratimod (trade name: Iremod), a novel small-molecule immunosuppressant, and MTX have also achieved positive results: iguratimod has an efficacy equivalent to MTX, but its safety is markedly superior. The main purpose of this study is to explore the efficacy and safety of combined etanercept and iguratimod in the treatment of active rheumatoid arthritis among Chinese population [5,6]. Related reports are scarce at home and abroad currently. |
Materials and Methods
|
Patient selection
|
| The patients selected aged between 20-60 years. 25 patients were males and 20 were females, all of whom were in line with the 1987 ACR RA classification criteria [7], and had active disease during screening. Exclusion criteria included: Individuals with severe injury of heart, liver, kidney or other vital organs, or have or have a history of blood or endocrine system lesions; pregnant or lactating women; individuals recently received etanercept treatment; individuals ineffective by MTX treatment; individuals recently received hormonal treatment; individuals during the acute or chronic infection period; or cancer patients or individuals with a family history of cancer. Clinical trial herein was conducted strictly following the Ethical Principles for Medical Research Involving Human Subjects in the Declaration of Helsinki, and was approved by the Ethics Committee of Dalian Friendship Hospital. All the patients selected have voluntarily signed an informed consent form, and the 45 patients enrolled were all from the Dalian Friendship Hospital. |
Trial methodology and duration of treatment
|
| Patients treated between July 2011 and July 2015 were selected, and divided into three groups based on therapeutic regimen. Patients in group A was treated with etanercept (subcutaneous injection at 25 mg twice weekly) in combination with MTX (initial dosage of 7.5 mg/week, and gradual increase of dosage to 15 mg/week from the fourth week). Patients in group B was treated with etanercept (subcutaneous injection at 25 mg twice weekly) in combination with iguratimod (oral administration at 25 mg twice daily). Patients in group C was treated with iguratimod alone (oral administration at 25 mg twice daily). |
| 15 patients were randomly selected from each therapeutic regimen group, who totaled 45 patients. And their treatment index data were collected prior to treatment as well as on the 12th, 24th, 36th and 52nd weeks after treatment, respectively. |
Clinical indices
|
| Prior to treatment and on the 12th, 24th, 36th and 52nd weeks after treatment, the following indices were assessed: clinical observational indices included: rest pain; duration of morning stiffness (minutes); joint swelling; joint tenderness, etc.; health assessment questionnaire (HAQ); patients' assessment of current disease situation; and physician's assessment of current disease situation. Improvement percentage of each index was calculated, and comprehensive assessment was conducted based on the improvement percentage of each index. |
Laboratory indices
|
| Prior to treatment and on the 12th, 24th, 36th and 52nd weeks after treatment, blood routine examination and biochemical test were performed, and erythrocyte sedimentation rate (ESR, Westergren method, normal value: male ≤ 15 mm/h, female ≤ 20 mm/h) and C-reactive protein (CRP, quantitative method) were determined. Prior to treatment and on the 12th, 24th, 36th and 52nd weeks after treatment, routine urine examination was carried out. Prior to treatment and on the 24th and 52nd weeks after treatment, rheumatoid factor (RF) test was performed. |
Safety indices
|
| Before enrollment, patients were checked for PPD, HbsAg antibodies, HCV-Ab and HCV antibodies. Laboratory indices required for monitoring during the trial included: blood routine, urine routine, liver and kidney functions, ANA and chest radiography. |
Imaging indices
|
| X-ray and MRI of both hands including wrist joints; and if necessary, other parts were also examined. Affected joints were scored using Sharp score. |
Efficacy assessment
|
| Therapeutic efficacy was assessed in strict accordance with the ACR20, ACR50, ACR70 criteria stipulated by the ACR [8]. ACR20 response was defined as 20% improvement in number of patients' tender and swollen joints, as well as 20% improvement in at least three of the following five measures: rest pain, activities of daily living, physician assessment, patient assessment and ESR or CRP. Using the same criteria, ACR50 and ACR70 were defined as 50% and 70% improvements, respectively. |
Adverse reactions
|
| During hospitalization, the occurrence time, severity, frequency, duration, handling measures and outcome of drug reactions such as fever, chills, rash and gastrointestinal reactions were recorded in detail, as well as continuation/ termination of treatment. |
Statistical analysis
|
| SPSS 13.0 was used for statistical analysis, and measurement data were described as x ± s. Efficacy between groups was compared by rank sum test, while the response rate between groups was compared by Fisher's exact test. P<0.05 was considered statistically significant. |
Results
|
Observational indices
|
| 45 patients were included into the trial, who were divided into groups A, B, and C, n = 15 in each group. It could be seen from the experimental results that the clinical symptoms and signs were all improved markedly than before treatment in all groups; moreover, improvements in rest pain, morning stiffness duration, joint swelling and joint tenderness after treatment in group A were significantly superior to the other two groups (P<0.05). After treatment, ESR, CRP and RF levels all decreased markedly in each group compared with before treatment; moreover, the decreases were more significant for group A (P<0.05). The results are shown in Table 1. |
Efficacy analysis
|
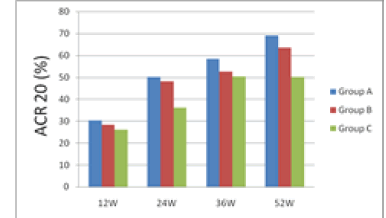
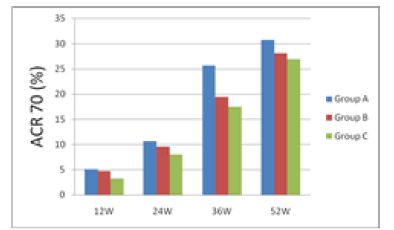
| 12 weeks after treatment, ACR20 response rate was 30.26% for group A, 28.21% for group B and 26.01% for group C; group A had the rate higher than groups B and C. 24 weeks after treatment, ACR20 response rates were nearly 50% for groups A and B, which were higher than group C's 36.25%. 36 weeks after treatment, ACR20, 50 and 70 of group A were all higher than groups B and C. 52 weeks after treatment, ACR20 response rate still remained significantly higher for group A than groups B and C. From the 12th week to the 52nd week, i.e. the end of the study, group A's ACR70 response rate was all along significantly higher than groups B and C, indicating that group A took effect fast and had a potency superior to groups B and group C. |
| The results are shown in Figures 1 and 2. |
| During the 52 weeks of treatment, groups A's ACR20 response rate was all along higher than groups B and C. Especially on the 12th, 24th, 36th and 52nd weeks, groups A's ACR20 response rates were 30.26%, 50.23% 58.47% and 69.12 %, respectively, demonstrating significantly better efficacy than group C's 26.01%, 36.19%, 50.36% and 50.24% (P<0.05). |
Comparison of efficacy indices
|
| 12 weeks after treatment, group A had more significant improvements in rest pain, duration of morning stiffness, joint swelling and joint tenderness indices compared with groups B and group C, suggesting that patients in group A had more prompt medical improvement and that drugs in A group took effects faster. After the completion of 52-week treatment, every index was improved obviously compared with before treatment in groups A, B and C. |
Adverse reactions
|
| Incidences of adverse reactions were 13.33%, 13.33% and 6.67% for groups A, B and C, respectively, showing no statistically significant differences between groups. Among them, the incidence of liver transaminase elevation was significantly higher for groups B and C than group A (P<0.05). Adverse reactions included gastrointestinal symptoms, dizziness, rash, etc., which were not significantly different among groups. No malignant tumor, death or serious adverse event was found in each group. |
Autoantibodies tests
|
| Autoantibodies test results before and after treatment showed that the ANA positive rate was 0.00% before treatment and 13.33% after treatment in group A, indicating that drugs in group A had no effect on autoimmunity or had little risk of increasing autoimmune diseases. |
Discusion
|
| Rheumatoid arthritis (RA) is a chronic, progressive disease with symmetric polyarthritis as the major manifestation. Deterioration of the condition can lead to joint damage or deformity, greatly affecting patients' quality of life, which is an important cause of labor incapacity and disability [9,10]. |
| Pathogenesis of RA is unclear at present, but some studies have suggested the association with tumor necrosis factor (TNF), which provides a new approach for treatment of RA as well. Etanercept is a novel biological agent, which can directly bind to TNF in vivo to cause cell lysis and activation of complement system. Research has found that TNF-α is a crucial synovial inflammatory cytokine, which can lead immune and inflammatory cells into the affected joints, thereby promoting the development and progression of the disease; this finding also provides a new approach for the treatment of RA [11]. |
| Etanercept is a novel biological agent, which can bind to soluble and transmembrane TNF-α to prevent their binding to cellular receptors, and induce the apoptosis of TNF-α-secreting immune cells. Its efficiency and safety in the treatment of RA have been confirmed by a large number of domestic and foreign literatures [12,13]. |
| MTX is a clinically recognized gold standard drug for RA at present, whose main mechanism is inhibition of dihydrofolate reductase and formyl transferase activities, and suppression of inflammatory cytokine production. Clinical studies have demonstrated its good short-term safety and tolerability, but long-term high-dose use is prone to cause blood system and liver damages [14,15]. |
| RA is a disease requiring long-term treatment. Studies have shown that 30%-64% of Chinese patients stop taking their medication due to serious adverse reactions. Therefore, the search for combination of a variety of drugs with different mechanisms of action but a synergistic effect has become a major topic in rheumatology for nearly 10 years. As a China's self-developed novel drug, iguratimod is a small-molecule immunosuppressant which can effectively inhibit RA, Ig levels, regulate B lymphocytes, promote osteoblast differentiation and facilitate bone reconstruction. Phase II and III MTX-controlled clinical trials have demonstrated its remarkable superiority in liver function and gastrointestinal side effects to MTX [16]. |
| Effectiveness and safety of combined etanercept and iguratimod for treatment of active rheumatoid have never been reported at home and abroad currently. This study compared the clinical efficacy between etanercept+MTX group, etanercept+iguratimod group and iguratimod-based empirical group by randomization, and observed the impact on radiographic joint progression and safety. Results revealed that combination of two drugs can improve RA symptoms early and slow the joint destruction, especially for those with poor MTX tolerance, the complementation of etanercept and iguratimod can achieve more satisfactory results. Combined application not only improved patient outcomes, but also reduced the dosage and side effects of individual drugs, thus ensuring the safety and compliance, improving the response rate while reducing the disability rate. |
Tables at a glance
|
 |
| Table 1 |
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |
References
|
- Kameda H, Okuyama A, Tamaru J, Itoyama S, Iizuka A, Takeuchi T. Lymphomatoidgranulomatosis and diffuse alveolar damage associated with methotrexate therapy in a patient with rheumatoid arthritis. Clinical Rheumatology 2007; 26: 1585-1589.
- Li L, Zhang X, Cui Y, Lu YY, Wang SX, Dong GF, Shi YZ, Luo RQ, Lei YX. Effect of recombinant human tumor necrosis factor receptor type II-Fc fusion protein antibody on cytokines and bone metabolism in patients with juvenile idiopathic arthritis.Zhonghua Yi XueZaZhi 2010; 90: 2205-2208.
- Wu QJ, Zhang ZL, Li ZB, Xu D, Li GT, Geng LF, Li MT, Wang Y, Zhu JJ, Hao YJ, Hui NL, Yang J, Cui XQ, Zhang XG, Zhao Y. An open-label, multicentric clinical trial to evaluate the efficacy and impact on bone metabolism of recombinant human tumor necrosis factor-α receptor II IgG Fc fusion protein with methotrexate in active rheumatoid arthritis: 24-week clinical and radiographic results from ReABLE study. Zhonghua Yi XueZaZhi 2010; 90: 2481-2485.
- Warzocha K, Robak T, Korycka A, Graczyk J, Pakulska W, Gaazka G, Kysik J. The influence of recombinant human tumor necrosis factor-alpha, alone and in combination with cyclophosphamide or methotrexate, on leukemia L1210 and normal hematopoiesis in mice. ArchivumImmunologiae et TherapiaeExperimentalis 1991; 39: 587-595.
- Keiichi T, Tomonobu Y, Masako H. Iguratimod for the treatment of rheumatoid arthritis in Japan. Expert Review of Clinical Immunology 2015; 11: 565-573.
- Koichi O, Yukio Y, Chisa O, Tsutomu K, Kenji T. Efficacy of the clinical use of iguratimod therapy in patients with rheumatoid arthritis. Modern Rheumatology 2015; 25: 235-240.
- Felson DT, Anderson JJ, Boers M. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995; 6: 727-735.
- Felson DT, Anderson JJ, Boers M. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995; 38: 727-735.
- Chen JW, Li XF, Zhang LY, Chen Z.Ru JL, Wang LY. The safety profiles of short-term etanercept combined with methotrexade and cyclophosphamide for treatment of rheumatoid arthritis. Chinese Remedies & Clinics 2008; 8: 455-456.
- Salehi E, Eftekhari R, Oraei M, Gharib A, Bidad K. MicroRNAs in rheumatoid arthritis. Clinical Rheumatology 2015; 34: 615-628.
- Feldmann M, Brennan FM, Maini RN. The role of cytokines in rheumatoid arthritis. Ann Rev Immunol 1996; 14: 397-440.
- Warzocha K, Robak T. Antileukemic effects of recombinant human tumor necrosis factor alpha (rh-TNF alpha) with cyclophosphamide or methotrexate on leukemia L1210 and leukemia P388 in mice. ActaHaematologicaPolonica 1992; 23: 55-62.
- Li L, Zhang X, Cui Y, Lu YY, Wang SX, Dong GF, Shi YZ, Luo RQ, Lei YX. Effect of recombinant human tumor necrosis factor receptor type II-Fc fusion protein antibody on cytokines and bone metabolism in patients with juvenile idiopathic arthritis. BIOCHEMICAL JOURNAL 2010; 90: 2205-2207.
- Esch MB, Mahler GJ, Stokol T, Shuler ML. Body-on-a-chip simulation with gastrointestinal tract and liver tissues suggests that ingested nanoparticles have the potential to cause liver injury. lab on a chip 2014; 14: 3081-3092.
- Bozkurt M, Bodakci MN, Turkcu G, Kuyumcu M, Akkurt M, Sula B, Em S, Oktayoglu P, Batmaz I, Yuksel H. Protective Effects of Carvacrol Against Methotrexate-induced Liver Toxicity in Rats.ActaChirurgicaBelgica 2014; 2014: 404-409.
- Hara M, Ishiguro N, Katayama K, Kondo M, Sumida T, MimoriTsuneyo, Soen Satoshi, Nagai K, Yamaguchi T, Yamamoto K. Safety and efficacy of combination therapy of iguratimod with methotrexate for patients with active rheumatoid arthritis with an inadequate response to methotrexate: An open-label extension of a randomized, double-blind, placebo-controlled trial. Modern Rheumatology 2014; 24: 410-418.
|

