Research Article - Biomedical Research (2017) Volume 28, Issue 17
Effects of recombinant human interferon α1b on Bcl-2 and c-myc expression in chronic myeloid leukemia and correlation with clinical treatment efficacy
Yuan He, Pengxiang Guo*, Xiaoli Du and Xue Fu
Department of Hematology, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, PR China
- *Corresponding Author:
- Pengxiang Guo
Department of Hematology
Guizhou Provincial People’s Hospital, PR China
Accepted on July 11, 2017
Abstract
Objective: Recombinant human interferon α1b is a clinical drug in treating certain viral diseases or malignant tumors, and is also widely used in treating leukemia, although its anti-tumor mechanism remains unclear. This study aimed to investigate the effect of recombinant human interferon α1b on expression of peripheral anti-tumor genes Bcl-2 and c-myc, and its correlation with clinical treatment efficacy.
Patients and Methods: A total of 95 chronic myeloid leukemia patients were recruited. Among all patients 46 received recombinant human interferon α1b and hydroxyurea, and 49 patients received hydroxyurea treatment only. Fluorescent qRT-PCR was used to measure mRNA expression of Bcl-2 and c-myc in peripheral blood of chronic myeloid leukemia patients. Treatment efficacy was evaluated from the aspect of hematology, molecular and cellular genetics. SPSS software was used to analyse the correlation between Bcl-2 and c-myc mRNA expression and clinical treatment efficacy.
Results: With elongated treatment time, Bcl-2 expression was significantly elevated after 6 w combined treatment, and c-myc expression was increased after 2 w combined treatment. Single hydroxyurea treatment group showed elevated levels after 6 w. Clinical treatment efficiency was positively correlated with Bcl-2 mRNA expression (r=0.541~0.623, p<0.01), and c-myc mRNA expression (r=0.645~0.612, p<0.01).
Conclusions: Recombinant human interferon α1b plays an important role in chronic myeloid leukemia treatment via facilitating Bcl-2 and c-myc expression.
Keywords
Recombinant human interferon α1b, Chronic myeloid leukemia, Bcl-2, c-myc.
Introduction
Leukemia is a type of malignant disease caused by abnormality of Hematological Stem Cells (HSCs), as colony cells lose differentiation or maturation ability for arresting at certain stage during development. Chronic Myeloid Leukemia (CML) is a hematological malignant tumor that severely threatens people’ health, and is also an acquired malignant clonal disease of HSCs with 1~2 per 100,000 incidence, occupying about 15% of newly diagnosed leukemia [1]. CML is frequently featured as Ph chromosome containing BCR-ABL1 fusion gene [2]. Pathogenesis of CML is still unclear and occupies about 3% of malignant tumors.
CML is an acquired clonal disease derived from HSCs, and is manifested as myeloid cell linage proliferation, peripheral leukocyte hyperplasia and spleen/liver enlargement [3], accompanying with Ph chromosome and BCR-ABL fusion gene in bone marrow cells [4]. Slow progression of CML disease makes insidious symptoms at early stage [5]. With disease progression, leukemia can impair normal hematological function of bone marrow and cause multiple related symptoms including anemia, refractory infection and hemorrhage. Current treatments for CML mainly focus on the chronic stage at early phase per disease pathogenesis mechanism. Common drugs for CML include imatinib, hydroxyurea, cytarabine and interferon [6,7]. By molecular target treatment and HSCs transplantation, drugs are also administrated to inhibit elevation of leukocytes. Before onset of molecular targeted drugs, interferon is the first choice of CML treatment. Recombinant human interferon α can obtain persistent cytogenetic remission of CML, thus elongating lifespan [8]. The treatment of CML using interferon has been accepted widely. Currently used interferon drugs include α2a and α2b interferon [9,10]. However, the treatment efficacy of interferon α1b on CML has not been clearly illustrated yet.
Bcl-2 gene was firstly separated from non-Hodgkin lymphoma, and is an important tumor suppressor gene playing important roles in suppressing cell apoptosis [11]. Previous study showed that most anti-tumor drugs could inhibit leukemia cell proliferation, induce cell apoptosis and suppress Bcl-2 expression [12]. C-myc gene participates in cell proliferation process, and facilitates cell apoptosis [13]. Tumorigenesis is the result of various genes and external factors. The single expression of Bcl-2 cannot induce tumor, but only with co expression with oncogene c-myc to induce tumorigenesis [14]. To further illustrate the important mechanism of interferon α1b in tumor treatment, this study investigated the effect of α1b interferon on Bcl-2 and c-myc expression.
Materials and Methods
Clinical information
A total of 95 CML patients admitted in hematology department of our hospital from May 2012 to October 2016 were recruited, including 57 males and 38 females. A retrospective control study was performed. 46 patients received combined treatment using both recombinant human interferon α1b and hydroxyurea, and 49 patients received hydroxyurea treatment only. Basic information including age and gender distribution between two treatment groups was analysed by statistical methods.
This study has been pre-approved by the ethical committee of Guizhou Provincial People’s Hospital. All subjects have signed the consent forms before recruitment in this study.
Diagnostic criteria
Inclusive criteria: (1) Anemia, uncomfortable in spleen area, hemorrhage, fatigue, body mass loss and low fever with elevated metabolic rate. (2) Leukocyte count>50 × 109/L, with occasionally higher than 500 × 109/L, hemoglobin<110 g/L indicating normochromic anemia, plus higher platelet at 1000 × 109/L. Blood smear showed granulocytes at various maturation stages, with predominant distribution at middle and late stage of promyelocytes, as less than 5% of primordial cells and less than 10% of primary granulocyte and early promyelocytes, increased count of basophilic and acidophilic granulocytes, and minor amounts of enucleated erythrocytes. (3) Fever, spleen swelling, bone pain, hemorrhage, extra-bone marrow edema infiltration with unknown reasons at acute phase. Meanwhile, a total of 38 healthy volunteers were recruited as the control group.
Treatment methods
Skin test was performed before using recombinant human interferon α1b. Subcutaneous or intramuscular injection was performed using α1b interferon (3000000 IU/d) (Kexing Biotech, China). Based on leukocyte count, hydroxyurea (Huarun Pharm, China) was orally applied (1-3 g/d) after 15~30 d of α1b interferon treatment, accompanied with leukocyte count. Drugs were stopped or decreased when leukocyte number returned to normal level.
mRNA extraction and mRNA expression level assay
Peripheral blood samples were collected and centrifuged to obtain serum. Trizol method was used to extract tissue RNA, whose content and purity were measured by spectrometry. RNA solution after quantification was analysed for integrity using 1% agarose gel electrophoresis. 1 μg RNA was used in reverse transcription of cDNA, following the manual instruction of real time fluorescent quantitative RT-PCR kit (TaKaRa, Japan). cDNA after reverse transcription was added into PCR system under following conditions: 95°C denature for 5 min, followed by 40 cycles each containing 95°C 15 s and 60°C 60 s. Amplification was performed in ViiA7 fluorescent quantitative PCR cycler. Each sample was performed in triplicates. Primers were synthesized by Jierui Biotech (China). Primer sequences were shown in Table 1.
| Gene | Primer sequence | Length |
|---|---|---|
| β-actin | Forward: 5’-AAACTGGAACGGTGAAGGTG-3’ | 119 pb |
| Reverse: 5’-AGTGGGGTGGCTTTTAGGAT-3’ | ||
| BCl-2 | Forward: 5’-CTCCCGCCGCCGCTACCGC -3’ | 125 pb |
| Reverse: 5’-CTGGGGCCGTACAGTTAC-3’ | ||
| c-myc | Forward: 5’-TTCTCTCCGTCCTCGGATTC -3’ | 116 pb |
| Reverse: 5’-GTAGTTGTGCTGATGTGTGGA -3’ |
Table 1. Bcl-2 and c-myc primer sequences.
Observation of treatment efficacy
After 2 and 6 w of treating CML, efficacy was observed in hematology, molecular biology and cytogenetics assays.
Hematology: Complete remission was defined as leukocyte<10 × 109/L, platelet<450 × 109/L and less than 0.05 of primordial cells in bone marrow.
Cytogenetics: Bone marrow samples were collected to analyse 20 cells at metaphase by R band visualization. Complete remission was defined with absence of Ph chromosome positive cells at metaphase.
Molecular biology: PCR was used to measure BCR/ABL mRNA level. Complete remission was identified when PCR results were negative based on international standard of BCRABL gene transcription level [15].
Statistical analysis
SPSS 16.0 software was used for statistical analysis of all data, of which measurement data were presented as mean ± Standard Deviation (SD). One-way Analysis of Variance (ANOVA) was used for comparison among multiple groups, followed by SNK-q test for between-group comparison. Enumeration data were analysed by chi-square test. Spearman approach was used for correlation analysis between two factors. A statistical significance was defined when p<0.05.
Results
General information of patients
By analyzing general information of CML patients and healthy volunteers in clinics, we found no significantly statistical difference of gender, age and Body Mass Index (BMI) between two groups, which were thus comparable (p>0.05, Table 2).
| Related index | CML | Control | P value |
|---|---|---|---|
| N | 95 | 38 | - |
| Gender | |||
| Male (N) | 57 | 22 | 0.832 |
| Female (N) | 38 | 16 | |
| Age (years) | 55.2 ± 13.2 | 59.6 ± 11.7 | 0.075 |
| BMI (kg/m2) | 20.5 ± 2.5 | 20.4 ± 2.1 | 0.828 |
Table 2. Generation information of participants.
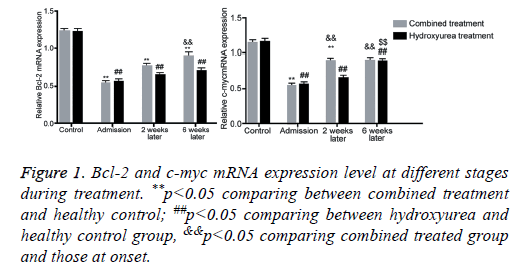
Bcl-2 and c-myc mRNA expression at different stages
Real-time fluorescent quantitative PCR has higher sensitivity and specificity than routine PCR. Using healthy population as the control group, Bcl-2 and c-myc mRNA expressions were quantified relatively. Results showed that with elongated treatment time, Bcl-2 mRNA level was significantly elevated after 6 w of combined treatment (p<0.05 compared with those at admission). C-myc level was significantly elevated at w 2 and w 6 of combined treatment group. Single use of hydroxyurea remarkably elevated expression level at 6 weeks after treatment (p<0.05 compared with those in admission, Figure 1). We further performed agarose gel electrophoresis on amplified products. Images were shown in Figures 2 and 3, with consistent results as those from quantification assay.
Comparison of efficacy among different treatment methods
After 2 or 6 w of combined treatment or hydroxyurea treatment, efficacy was analysed from hematology, cytology and genetics. Results showed that combined treatment of recombinant human interferon α1b plus hydroxyurea had better efficacy than singly treatment using hydroxyurea from all perspectives, as shown in Table 3.
| Group | CHR | CMR | CCyR |
|---|---|---|---|
| Combined treatment | 86.32%** | 39.55%** | 57.90%** |
| Hydroxyurea | 60.86% | 20.14% | 37.54% |
Note: CHR: Complete Hematology Reaction; CMR: Complete Molecular Response; CCyR: Complete Cytogenetics Response. **p<0.05 compared to hydroxyurea treatment group.
Table 3. Comparison of treatment efficacy among different methods.
Correlation between Bcl-2/c-myc mRNA expression level and treatment efficacy
Pearson analysis was performed to reveal the correlation between Bcl-2 or c-myc mRNA expression and clinical treatment efficacy. Results showed positive correlation of treatment efficacy with Bcl-2 mRNA expression (r=0.541~0.623, p<0.01), and c-myc mRNA expression (r=0.645~0.612, p<0.01, Table 4).
| Treatment efficacy | BCl-2 | c-myc | ||
|---|---|---|---|---|
| r | P | r | P | |
| Hematology | -0.541 | P<0.01 | -0.621 | P<0.01 |
| Molecular biology | -0.562 | P<0.01 | -0.645 | P<0.01 |
| Cytogenetics | -0.623 | P<0.01 | -0.612 | P<0.01 |
Table 4. Correlation between Bcl-2/c-myc mRNA expression and treatment efficacy.
Discussion
Hydroxyurea exerts its treatment effects via inhibiting nucleic acid reductase to interfere with DNA synthesis on S phase [16,17]. It usually functions on late stage of hematological system, thus having minor suppression on bone marrow. Interferon α1b has property of anti-virus and suppression of cell proliferation without selectivity. Therefore, it is more effective to use both hydroxyurea and interferon, but without clearly illustrated functional mechanisms.
Results of this study showed lower expression of tumor suppressor gene Bcl-2 and c-myc in CML patients compared with control population, consistent with previous studies [18,19]. After using both hydroxyurea and interferon for treating CML patients, mRNA expression of Bcl2 and c-myc was elevated with extended treatment time, indicating that hydroxyurea and recombinant α1b could exert treatment effects via facilitating expression of tumor suppressor gene. However, compared with single use of hydroxyurea, combined treatment can effectively accelerate Bcl-2 and c-myc up-regulation. In vitro study of interferon α-bone marrow cell co-culture showed that interferon α could affect cell biological behavior via modifying Bcl-2 or c-myc gene expression, thus alleviating disease severity. The results of in vivo study were consistent with those in vitro studies. Interferon α suppressed BCR/ABL gene expression in CML leukemia cell lineage [20]. BCR/ABL fusion gene can exert anti-apoptotic effects via enhancing expression of anti-apoptotic gene Bcl-2.
Combined treatment using both recombinant human interferon α1b and hydroxyurea had better efficacy than hydroxyurea alone from perspectives of cytology, hematology and genetics. Combing with previous study data, using both α interferon and imatinib for treating CML had better efficacy than single use of imatinib in both low risk and moderate risk groups, but not in those high risk patients [21]. Alpha-interferon had certain diagnostic values for hairy cell leukemia and CML [22]. However, analysis of different CML grades was not performed in the present study and requires further investigation in the future. Interestingly, our study showed positive correlation between Bcl-2/c-myc expression and clinical treatment efficacy as demonstrated by improved treatment efficacy along with elevated Bcl-2 or c-myc expression. However, due to limited numbers of patients enrolled in the present study, large cohort clinical studies are required to confirm these findings in the future.
Conclusion
Combined treatment of interferon and hydroxyurea had better efficacy than single use of hydroxyurea from cytology, hematology and genetics perspectives. Both treatments elevated mRNA expressions of Bcl-2 and c-myc. Therefore, recombinant human interferon α1b has treatment effects on CML.
References
- Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2016 update on diagnosis, therapy, and monitoring. Am J Hematol 2016; 91: 252-265.
- Heisterkamp N, Stam K, Groffen J, de Klein A, Grosveld G. Structural organization of the bcr gene and its role in the Ph translocation. Nature 1985; 315: 758-761.
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell 2000; 100: 157-168.
- Vinhas R, Cordeiro M, Pedrosa P, Fernandes AR, Baptista PV. Current trends in molecular diagnostics of chronic myeloid leukemia. Leuk Lymphoma 2016; 1-14.
- Kantarjian H, OBrien S, Jabbour E, Garcia-Manero G, Quintas-Cardama A, Shan J, Rios MB, Ravandi F, Faderl S, Kadia T, Borthakur G, Huang X, Champlin R, Talpaz M, Cortes J. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood 2012; 119: 1981-1987.
- Jabbour E. Chronic myeloid leukemia: first-line drug of choice. Am J Hematol 2016; 91: 59-66.
- Baccarani M, Castagnetti F, Gugliotta G, Palandri F, Rosti G. Treatment recommendations for chronic myeloid leukemia. Mediterr J Hematol Infect Dis 2014; 6: e2014005.
- Talpaz M, Hehlmann R, Quintas-Cardama A, Mercer J, Cortes J. Re-emergence of interferon-alpha in the treatment of chronic myeloid leukemia. Leukemia 2013; 27: 803-812.
- Takeuchi K. Analysis of IFN-alpha inducible genes in human chronic myelogenous leukemia cell line KT-1. Rinsho Ketsueki 2003; 44: 65-69.
- Yassin MA, Kohla S, Al-Sabbagh A, Soliman AT, Yousif A, Moustafa A, Battah AA, Nashwan A, Al-Dewik N. A case of chronic neutrophilic leukemia successfully treated with pegylated interferon alpha-2a. Clin Med Insights Case Rep 2015; 8: 33-36.
- Willimott S, Wagner SD. Post-transcriptional and post-translational regulation of Bcl2. Biochem Soc Trans 2010; 38: 1571-1575.
- Chanvorachote P, Nimmannit U, Stehlik C, Wang L, Jiang BH, Ongpipatanakul B, Rojanasakul Y. Nitric oxide regulates cell sensitivity to cisplatin-induced apoptosis through S-nitrosylation and inhibition of Bcl-2 ubiquitination. Cancer Res 2006; 66: 6353-6360.
- Lin CY, Loven J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 2012; 151: 56-67.
- Milner AE, Grand RJ, Vaughan AT, Armitage RJ, Gregory CD. Differential effects of BCL-2 on survival and proliferation of human B-lymphoma cells following gamma-irradiation. Oncogene 1997; 15: 1815-1822.
- Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, Baccarani M, Cortes J, Cross NC, Druker BJ, Gabert J, Grimwade D, Hehlmann R, Kamel-Reid S, Lipton JH, Longtine J, Martinelli G, Saglio G, Soverini S, Stock W, Goldman JM. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 2006; 108: 28-37.
- Kennedy BJ, Yarbro JW. Metabolic and therapeutic effects of hydroxyurea in chronic myeloid leukemia. JAMA 1966; 195: 1038-1043.
- de Lima PD, Cardoso PC, Khayat AS, Bahia Mde O, Burbano RR. Evaluation of the mutagenic activity of hydroxyurea on the G1-S-G2 phases of the cell cycle: an in vitro study. Genet Mol Res 2003; 2: 328-333.
- Cramer K, Nieborowska-Skorska M, Koptyra M, Slupianek A, Penserga ET, Eaves CJ, Aulitzky W, Skorski T. BCR/ABL and other kinases from chronic myeloproliferative disorders stimulate single-strand annealing, an unfaithful DNA double-strand break repair. Cancer Res 2008; 68: 6884-6888.
- Delgado MD, Leon J. Myc roles in hematopoiesis and leukemia. Genes Cancer 2010; 1: 605-616.
- Yanagisawa K, Yamauchi H, Kaneko M, Kohno H, Hasegawa H, Fujita S. Suppression of cell proliferation and the expression of a BCR-ABL fusion gene and apoptotic cell death in a new human chronic myelogenous leukemia cell line, KT-1, by interferon-alpha. Blood 1998; 91: 641-648.
- Bohn JP, Gastl G, Steurer M. Long-term treatment of hairy cell leukemia with interferon-α: still a viable therapeutic option. Memo 2016; 9: 63-65.
- Burchert A, Saussele S, Eigendorff E, Muller MC, Sohlbach K, Inselmann S, Schutz C, Metzelder SK, Ziermann J, Kostrewa P, Hoffmann J, Hehlmann R, Neubauer A, Hochhaus A. Interferon alpha 2 maintenance therapy may enable high rates of treatment discontinuation in chronic myeloid leukemia. Leukemia 2015; 29: 1331-1335.