Research Article - Biomedical Research (2017) Volume 28, Issue 6
Effects of pulsed electromagnetic field on mineral density, biomechanical properties, and metabolism of bone tissue in heparin-induced osteoporosis in male rats
Müge Çina Aksoy*, Olgun Topal, Hatice Varol Özkavak, Duygu Kumbul Doğuç, Azime Aslıhan Ilhan and Selçuk Çömlekçi
Department of Oral Maxillofacial Surgery, Faculty of Dentistry, Süleyman Demirel University, Isparta, Turkey
- *Corresponding Author:
- Müge Çina Aksoy
Department of Oral Maxillofacial Surgery
Faculty of Dentistry
Süleyman Demirel University, Turkey
Accepted on November 10, 2016
Abstract
Owing to the aging world population, medical problems due to old age significantly burden healthcare and economic systems. A major health problem in elderly individuals is loss of bone mass due to osteoporosis, a popular biomedical research subject. Current medical approaches to osteoporosis include pharmacological treatment options, with undesirable adverse effects resulting from many drugs. Alternative methods have been investigated to prevent bone-mass loss or increase bone mass. For example, pulsed electromagnetic field applications are well-studied modalities with varying results. Most studies on PEMF were conducted on postmenopausal women or ovariectomized rats, but epidemiological data suggest that osteoporosis in males is as crucial as in females. This study is the first to evaluate the PEMF effects on heparin-induced osteoporosis in male rats. We induced osteoporosis by administering heparin injections to rats for 33 days, and applied PEMF at a 0.8-mT intensity and 7.3-Hz frequency for 1 h daily for 4 weeks starting from the 35th day of the experiment. The response to pulsed electromagnetic field in bone tissue was evaluated using bone mineral density results, biomechanical measurements, and receptor activator of nuclear factor κB/ RANK ligand/osteoprotegerin evaluations. We observed that PEMF did not significantly affect bone mass restoration in osteoporosis models. The biomechanical measurements revealed inconclusive findings in favor of pulsed electromagnetic field application. We concluded that the presence of osteoporosis and the biological background from which osteoporosis develops contribute to the PEMF effects, and hormonal exposure in females may be related to the favorable effects of PEMF on bone remodeling.
Keywords
Pulsed electromagnetic field, Osteoporosis, Bone remodelling, OGP, RANK, RANKL.
Introduction
Globally, life expectancy has been increasing owing to improvements in medical science, comprehension of healthy living, and many other medico-social issues. The major health consequence of old age in a community is the increased incidence of chronic diseases and geriatric syndromes [1]. Aging significantly affects the composition of tissues, and bone density begins to decrease in the fourth decade of life [2]. Decreased bone density leads to increased osteoporosis (OP) in elderly individuals, which can be interpreted as a global public health problem owing to osteoporotic fractures and the associated mortality, morbidity, and economic burden [3]. Most osteoporotic fractures include hip, vertebrae, and distal forearm fractures, but recent data show that the risk of all types of fractures significantly increases in individuals with decreased bone mineral density (BMD) [4]. In addition, non-spine non-hip osteoporotic fractures also pose a substantial burden to health and economy systems owing to their frequency being 10-20 times higher than those of other fractures [5]. In general, OP is thought to affect women, but increasing attention has been given to male OP [6] because recent data show that elderly males are also prone to mortality and morbidity related with increased osteoporotic fractures. According to a report, “As many as one in four men over the age of 50 will develop at least one OP-related fracture in their lifetime” [7]. Hence, OP is now the most prevalent bone diseases [8] and one of the major concerns of public health in the elderly, which affects both sexes.
OP significantly decreases the resistance of bones to trauma, and daily activities can easily result in a fracture [9]. Hence, both protective and therapeutic interventions should be applied to patients with OP to maintain their quality of life. However, current clinical approaches to OP are challenging in the medical sciences field [10]. The established interventions cover a wide range of applications, mostly pharmacological, which fall in two categories, i.e., promoters of bone formation and inhibitors of bone resorption, but each have their own adverse effects [11,12]. Because of these undesired consequences of drug applications, alternative methods have been investigated for a long period of time. One of the regimens proposed to be a safe and effective physiotherapy modality is the use of pulsed electromagnetic field (PEMF) [10]. The first application of PEMF was in 1974 as a clinical example of fracture healing [13], which subsequently led to increased interest in this method. Current experimental and clinical data suggest that PEMF provides beneficial effects in bone fracture healing [14], osteogenesis [15], and enhancement of the BMD [16]. PEMF has been attracting interest in bone remodeling and has been under investigation as an alternative approach for treating OP for approximately four decades because of the global burden on health and economic systems and the adverse pharmacotherapy-related effects of this medical condition, and a substantial amount of data regarding its effects on bone tissue is available. The basic mechanism of PEMF that results in these effects is its influence on bone metabolism [17]. However, the depths of signaling pathways and relevant mechanisms that are responsible for the effects of PEMF are still unclear [10].
The bone density depends on the balance of osteoblastic and osteoclastic activities, which control the formation or resorption rates of bone tissue in an organism [18]. According to the current data on bone remodeling, osteoclastic regulation is strongly related to a pathway that includes three main components: a receptor activator of nuclear factor κB (RANK), RANK ligand (RANKL), and osteoprotegerin (OPG) [19]. The interaction of RANK with RANKL yields enhanced osteoclastic activity [20], whereas RANK-OPG interaction inhibits osteoclastic activity and subsequent bone resorption [19]. Current data about the effects of PEMF on OP are mainly obtained from studies conducted on postmenopausal women or ovariectomized animal models. As the current epidemiological data suggest that OP in males is as important as that in women, in relation to the medical and economic burden, we aimed to evaluate the BMD, biomechanical properties, and OPG/ RANK/RANKL pathways in an experimental heparin-induced OP model in male rats.
Materials and Methods
Animals, groups, and intervention
A total of 26 mature (mean age of 12 months) Sprague-Dawley male rats were used in the study. Male rats were used to eliminate the effects of menstrual cycles, which occur for female rats. The subjects were kept in 50 × 50 × 15 cm3 boxes with ventilation holes, light and temperature control, and unrestricted access to food and water. The environment was stabilized to a temperature and humidity of 21-22°C and 50%-55%, respectively. The nutrition was maintained ad libitum with pellet food and running water. All the rats were randomly categorized into three groups: eight rats were subjected to OP induction by heparin and subsequent PEMF application (Heparin+PEMF), eight rats were subjected only to OP induction by heparin (Heparin), and the remaining ten rats formed the control group (Control). Of them one rat was died due to complications arising during anesthesia and excluded from the study. All the rats were killed at the end of the experiments (63rd day) by exsanguination under anesthesia. The study design and relevant procedures were approved by the author’s institutional review board and that animal care complied with the guidelines of the authors' institution or any national law on the care and use of laboratory animals.
Heparin-induced OP procedure
The heparin application procedure was designed according to data in the literature for heparin-induced OP progression. A daily single dose of 2 IU/g of heparin (Mustafa Nevzat ?laç Sanayii A. ?., ?stanbul, Turkey) was subcutaneously administered to rats via an insulin injector for 33 days by the same researcher. Every milliliter of heparin solution included 5000 IU of heparin sodium. The heparin doses were regulated according to the body weights of the rats, and a mean of ~0.08 mL heparin was diluted in 0.92 mL of water.
PEMF application
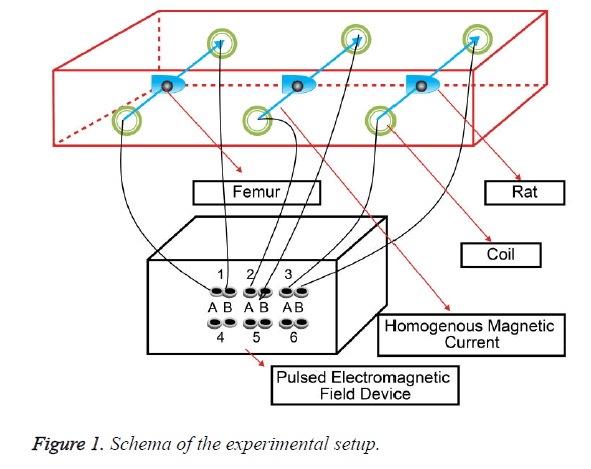
The rats were exposed to PEMF for 1 h daily for four weeks, starting from the 35th day, at a 0.8-mT intensity and (440 rpm) 7.3 Hz frequency, which was provided by a PEMF device, namely, EMTS 3005S (Biomedsa, Isparta, Turkey), and the movement of the subjects was restricted by a restrainer. Helmholtz coils consisting of 200 insulated copper wire windings were used to cretae a uniform magnetic field. These coils are actually a system of corresponding bobbins with diameter “a” and horizontal distance “b” (Figure 1).
The experimental mechanism was designed de novo by the researchers for the study. The coils that connected to the lead terminals of the device were prepared using 1-mm isolated copper wires that could be coiled to a diameter of 6 cm, and they were placed on the corresponding faces of a rectangular box 10 cm away from each other. The device was oriented in such a way so that the vector magnetic field, that is generated by the passage of electric current through the coils, is directed perpendicularly to the femurs. Five restrainers were placed in two 100 × 10 × 15 cm3 wooden boxes, and bobbins were placed on the outer faces of the boxes. The rats were arranged in restrainers that kept them immobile ensuring, such that they were perpendicular to the homogenous magnetic current vector. To eliminate the environmental factors that could potentially affect the PEMF intensity, the process was conducted in a fully isolated magnetic field room. Frequency and intensity measurements were performed using a digital Gauss/Teslameter (Unilab, Blackburn, England) before and during the experiment to assure the reliability of the device and standardization of the pulsed magnetic field. The measurements on the femurs were performed after killing the rats and separating the soft tissues completely from the bones.
BMD
The bone mineral compositions and densities of the femur samples were evaluated using dual energy X-ray absorptiometry (DEXA; Hologic Wi[S/N 84305], Marlborough, USA) using a DEXA device with a high-resolution imaging capability for small experimental animals.
Biomechanical evaluations
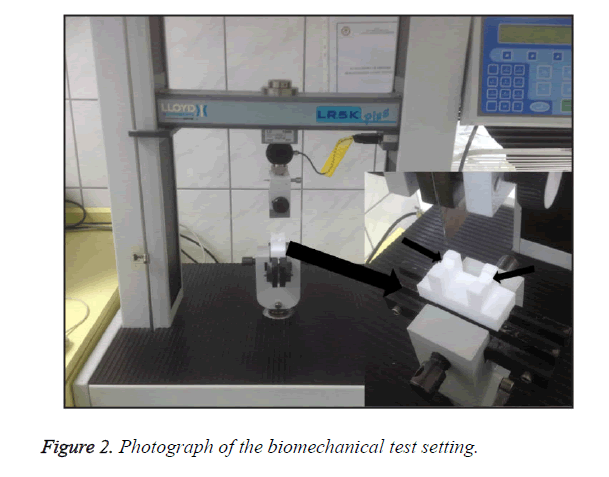
The femur samples were preserved in physiologic solutions at -20°C and thawed in a humid environment before the measurements. After the samples reached a temperature of 21-22°C, they were immersed in isotonic saline at 37°C and tested in the laboratories of the Suleyman Demirel University Textile Engineering Department. Three-point bending tests were performed at 21-22°C using a LR5K Plus device (LLOYD Instruments Ltd., Farnham, UK) that was equipped with a custom-designed apparatus, which keeps the femur heads on top and prevents dislocation during the measurement (Figure 2). The effective spans, in a range of 13-15 mm depending on the length of the femurs, were individually determined for each subject. A single load was applied at the symmetrical midpoint of the femurs. Additionally, the mean cortical thicknesses, anterior-posterior diameters, and inner-outer diameters were measured using a digital caliper, which has a sensitivity of 1/50 mm. The maximum load was determined as the one that caused fracture. The maximum load was used to calculate the bending stress (endurance) of the femur according to the following equation:
e=(Me × c)/I
e: Maximum bending stress (N/mm2)
Me: Maximum bending momentum (N?mm)
c: Distance from the center to the outermost surface on the transvers section at the point of bending load application (mm)
I: Cross-sectional moment of inertia of the bone (mm4)
The area moment of inertia was calculated according to the following equation by assuming that the cross sections of the femurs were ellipsoidal:
I=[ab3 - ((a-2t)(b-2t)3)] / 64
a: Cross-sectional length of the inner-outer lateral axis (mm)
b: Cross-sectional length of the anterior-posterior axis (mm)
t: Mean cortical thickness (mm)
a and b values were measured from the center of the bones
The fracture sections of the femurs were evaluated by stereomicroscopy (Leica S4E, Leica Mikrosysteme Vertrieb GmbH, Wetzlar, Germany), and the findings obtained in the mechanical tests were confirmed by these fracture patterns.
Biochemical analyses
The blood samples for biochemical analyses were collected from the vena cava when the rats were killed (63rd day). The samples were kept at 21-22°C for 0.5 h and then centrifuged for 10 min at 4500 rpm (Nuve NF1200R, Turkey). The blood serums were portioned (~0.8-1 mL) and kept at -80°C until the analyses were performed. Commercial rat-specific enzyme-linked immunosorbent assay kits were used to determine the levels of RANK, RANKL, and OPG in the serum samples as recommended by the producer (SunRed, Sunred Biological Technology Co., Shanghai, China). The standards and samples were run in duplicate in each assay, and a standard curve plot was used to determine the concentrations of RANK, RANKL, and OPG for each sample. The sensitivities of the RANK, RANKL, and OPG were 0.137 ng/mL, 0.086 pmol/L, and 0.044 ng/mL, respectively.
Statistical analyses
Statistical analyses were performed with SPSS 15 (SPSS Inc., Chicago, IL). Multi-group comparisons were performed using either an ANOVA or a Kruskal-Wallis test when the normal distribution assumptions were met or not met, respectively. If the ANOVA test was used, the post-hoc pairwise comparisons were made with a Tukey test. The correlations between the numerical data were evaluated using Pearson correlation analyses. A type-I error level of 5% was considered as the statistical significance level in the analyses.
Results
The results of the bone densitometry and biomechanical evaluations are listed in Table 1. The lowest and highest BMD values were observed for the heparin and control groups, respectively (p<0.001). The pairwise post-hoc comparisons revealed that both the intervention groups had BMD values significantly lower than those of the controls, but they were statistically similar to each other. Meanwhile, the bending strength in all three groups was similar (p=0.847). The levels of the biomarkers of the bone metabolism are listed in Table 2. According to these measurements, the OPG (p=0.687) and RANK (p=0.593) levels are similar between the study groups, but the RANKL levels significantly differ between the intervention and control groups (p=0.006), and the control group has the lowest RANKL levels. PEMF application was found to have no significant effect on the RANKL levels, and the heparin + PEMF and heparin-only groups did not differ with respect to this biomarker. The correlations between the clinical parameters of the study are listed in Table 3. According to the assessments, the only correlated parameters are the bending strength and RANKL (r=-0.442, p=0.027), and a negative and moderate correlation exists between these two measurements. No other statistically significant correlations exist between the remaining parameters.
| Heparin+PEMF | Heparin | Control | p | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| [Min-Max] | [Min-Max] | [Min-Max] | ||
| BMD | 0.266 ± 0.020c | 0.252 ± 0.012c | 0.285 ± 0.007a,b | <0.001 |
| [0.239-0.301] | [0.226-0.263] | [0.273-0.294] | ||
| Bending Strength | 189.9 ± 36.6 | 180.9 ± 30.0 | 188.0 ± 32.0 | 0.847 |
| [153.9-246.4] | [128.7-228.6] | [130.7-230.0] | ||
| a:Different from Heparin+PEMF group, b:different from Heparin group, c:different from control group, BMD: Bone Mineral Density. | ||||
Table 1. Results of bone densitometry and bending strength.
| Heparin+PEMF | Heparin | Control | p | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| [Min-Max] | [Min-Max] | [Min-Max] | ||
| OPG | 2.902 ± 0.642 | 3.067 ± 0.414 | 3.147 ± 0.660 | 0.687 |
| [2.098-3.937] | [2.210-3.452] | [2.382-4.324] | ||
| RANK | 6.450 ± 0.949 | 6.473 ± 0.731 | 6.146 ± 0.478 | 0.593 |
| [5.334-7.962] | [5.425-7.531] | [5.713-7.178] | ||
| RANKL | 5.000 ± 0.687c | 4.871 ± 0.316c | 4.302 ± 0.224a,b | 0.006 |
| [4.198-5.954] | [4.479-5.331] | [4.034-4.653] | ||
| a: Different from Heparin+PEMF group, b: different from Heparin group, c: different from control group, OPG: Osteoprotegerin; RANK: Receptor Activator of Nuclear Factor kappa-B; RANKL: RANK ligand | ||||
Table 2. Results of bone densitometry and biomechanical evaluations.
| BMD r (p) | Bending Strength r (p) | OPG r (p) | RANK r (p) | |
|---|---|---|---|---|
| Bending Strength | -0.172 (0.411) | |||
| OPG | 0.087 (0.678) | -0.103 (0.624) | ||
| RANK | -0.198 (0.342) | -0.083 (0.692) | 0.026 (0.902) | |
| RANKL | -0.197 (0.346) | -0.442 (0.027) | 0.079 (0.708) | 0.383 (0.059) |
| BMD: Bone Mineral Density; OPG: Osteoprotegerin; RANK: Receptor Activator of Nuclear Factor kappa-B; RANKL: RANK ligand | ||||
Table 3. Correlations between clinical parameters of the study.
Discussion
The present study evaluated the effects of PEMF on an experimental OP model in male rats by BMD and biomechanical measurements and checked for supportive findings in the measurements of biomarkers of bone metabolism. Our results showed that PEMF did not significantly affect the bone with regard to the BMD or bending strength. The bone metabolism indicators were similar for the groups with and without PEMF application. Additionally, the consistency between the biomechanical evaluations and metabolic markers was assessed via correlation analyses, and the results showed that the bending strength was negatively correlated with the RANKL, which confirms the role of this metabolic indicator in osteoclastic activity. Some of the current evidence suggests that PEMF has favorable effects on the prevention of bone mass loss [8,21], but others have not indicated these favorable effects [22,23], similar to the case with our study.
In a randomized controlled clinical study of postmenopausal women, Liu et al. reported that PEMF application was as efficacious as alendronate in the treatment of OP [24]. Elsisi et al. reported the beneficial effects of PEMF on BMD increase in elderly women between 60 and 70 years [2]. In an experimental study, Chang and Chang reported that PEMF inhibited the progression of OP in ovariectomized rats [8]. Another experimental study by Shen et al. reported the efficiency of PEMF in suppressing bone mass loss in rats with disuse OP [15]. The main difference between the studies with and without the beneficial outcome of PEMF is the application procedure of electromagnetic stimulation. Because the basic principle of creating a secondary electrical field in a bone tissue to produce metabolic effects strictly depends on the characteristics of the primary field generated by an external coil [25], different results will be obtained depending on the factors that influence this mechanism. The intensity and frequency of the PEMF stimuli, duration of exposure, and internal characteristics of the subjects are the main factors that affect the results. We used a stimulus with a 0.8-mT intensity and 7.3-Hz frequency, and the rats were exposed to an electromagnetic field for 1 h daily. In a study by Chang and Chang, which reported the beneficial effects of PEMF, rats were exposed to PEMF with an 8-G single-pulse waveform for 8 h per day [8]. Another study by Jing et al. reported trabecular thickening in ovariectomized rats when they were exposed to pulsed bursts with a maximum of 9.6 G for 6 h per day [21]. Both the intensity and duration of exposure in our study are different from those in these studies with positive findings. Hence, we can conclude that the duration of exposure to PEMF should be more than that in our study to obtain a positive effect.
Meanwhile, we consider the experimental model of OP when interpreting the results. Rats are known to be some of the most widely used animals in experiments for OP models owing to their skeletal structure, lifecycles, and ease in production [26]. Heparin-induced OP is one of the most prevalent methods used in experimental studies of OP, and some of the previous studies reported that the heparin injection for 28 days causes irreversible secondary OP [27]. However, to the best of our knowledge, no previous data are available on the effects of PEMF on the heparin-induced OP model for rats. Most of the experimental models are designed for ovariectomized rats or based on clinical studies on postmenopausal women. Hormonal factors in the progression of OP may influence the subsequent metabolic responses to external environmental stimuli. Another point to consider is the sex of the subjects. We used male rats in the experiments to eliminate hormonal factors related to the menopausal cycle in rats. This result also implies the importance of hormonal factors in OP.
In relation to the metabolic modification of OP, another significant pathway is an OPG/RANK/RANKL system that directly influences the balance between osteoblastic and osteoclastic activities. OPG is one of the most potent antiresorptive agents known, and it can be used to determine the direction of bone metabolic activity [26]. Its production is controlled by a number of cytokines and hormones, including sex steroids [28]. The presence of estrogen in an organism stimulates OPG production [29], and testosterone inhibits the production of this osteoblastic molecule [30]. An increased RANKL/OPG ratio results in bone resorption, and the absence of estrogen or presence of testosterone is a strong precipitator of this imbalance in osteoclastic activity. Our results confirm this finding, as we found that the RANKL levels were significantly higher in osteoporotic rats than in the controls. However, there was no significant difference in the RANKL level for the rats with and without PEMF application, which may be due to the fact that the efficiency of the PEMF application strongly depends on the nature of OP in living organisms. In addition, most of the data about the OPG/ RANK/RANKL system are gathered from postmenopausal subjects [30], which may not reflect the exact mechanisms in our OP model.
Conclusion
To the best of our knowledge, the results reported herein are the first on the effects of PEMF on an experimental heparin-induced OP model without a background of estrogen exposure. From the evaluations, we found that PEMF has no significant effect on bone metabolism in male rats, which is suggestive of the importance of the presence of estrogen in the organism in order to obtain beneficial results of PEMF treatment for OP. This study was carried out on male rats only within the limitations of our study. Further studies are required on two groups of rats, i.e., males and females, under the same conditions to better explain the effects of PEMF treatment for OP. As we focus on the densitometric and mechanical features of femurs in our study, further studies are required using basic μCT as well as histomorphometric analyses.
Acknowledgement
This study (Rats, heparin and PEMF device) was granted by The Scientific and Technological Research Council of Turkey (TUBITAK) 3001-Starting R&D Projects Funding Program, Project Number: 114S071.
References
- Fiatarone Singh MA. Benefits of exercise and dietary measures to optimize shifts in body composition with age. Asia Pac J Clin Nutr 2002; 11 Suppl 3: S642-652.
- Elsisi HF, Mousa GS, ELdesoky MT. Electromagnetic field versus circuit weight training on bone mineral density in elderly women. Clin Interv Aging 2015; 10: 539-547.
- Orsini LS, Rousculp MD, Long SR, Wang S. Health care utilization and expenditures in the United States: a study of osteoporosis-related fractures. Osteoporos Int 2005; 16: 359-371.
- Cooper C, Cole ZA, Holroyd CR, Earl SC, Harvey NC, Dennison EM, Melton LJ, Cummings SR, Kanis JA; IOF CSA Working Group on Fracture Epidemiology. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporosis Int 2011; 22: 1277-1288.
- Shi N, Foley K, Lenhart G, Badamgarav E. Direct healthcare costs of hip, vertebral, and non-hip, non-vertebral fractures. Bone 2009; 45: 1084-1090.
- Willson T, Nelson SD, Newbold J, Nelson RE, LaFleur J. The clinical epidemiology of male osteoporosis: A review of the recent literature. Clin Epidemiol 2015; 7: 65-76.
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17: 1726-1733.
- Chang K, Chang WH. Pulsed electromagnetic fields prevent osteoporosis in an ovariectomized female rat model: A prostaglandin E2-associated process. Bioelectromagnetics 2003; 24: 189-198.
- Tabrah F, Hoffmeier M, Gilbert F, Batkin S, Bassett CA. Bone density changes in osteoporosis-prone women exposed to pulsed electromagnetic fields (PEMFs). J Bone Miner Res 1990; 5: 437-442.
- Zhou J, He H, Yang L, Chen S, Guo H. Effects of pulsed electromagnetic fields on bone mass and Wnt/β-catenin signaling pathway in ovariectomized rats. Arch Med Res 2012; 43: 274-282.
- Gallacher SJ, Dixon T. Impact of treatments for postmenopausal osteoporosis (bisphosphonates, parathyroid hormone, strontium ranelate, and denosumab) on bone quality: A systematic review. Calcif Tissue Int 2010; 87: 469-484.
- Rizzoli R, Reginster JY, Boonen S, Bréart G, Diez-Perez A. Adverse reactions and drug-drug interactions in the management of women with postmenopausal osteoporosis. Calcif Tissue Int 2011; 89: 91-104.
- Bassett CA, Pawluk RJ, Pilla AA. Acceleration of fracture repair by electromagnetic fields. A surgically noninvasive method. Ann N Y Acad Sci 1974; 238: 242-262.
- Assiotis A, Sachinis NP, Chalidis BE. Pulsed electromagnetic fields for the treatment of tibial delayed unions and nonunions. A prospective clinical study and review of the literature. J Orthop Sur Res 2012; 7: 24.
- Shen WW, Zhao JH. Pulsed electromagnetic fields stimulation affects BMD and local factor production of rats with disuse osteoporosis. Bioelectromagnetics 2010; 31: 113-119.
- Eyres KS, Saleh M, Kanis JA. Effect of pulsed electromagnetic fields on bone formation and bone loss during limb lengthening. Bone 1996; 18: 505-509.
- Spadaro JA, Bergstrom WH. In vivo and in vitro effects of a pulsed electromagnetic field on net calcium flux in rat calvarial bone. Calcif Tissue Int 2002; 70: 496-502.
- Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA. Local communication on and within bone controls bone remodeling. Bone 2009; 44: 1026-1033.
- Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 2008; 473: 139-146.
- Zhou J, Chen S, Guo H, Xia L, Liu H. Pulsed electromagnetic field stimulates osteoprotegerin and reduces RANKL expression in ovariectomized rats. Rheumatol Int 2013; 33: 1135-1141.
- Jing D, Shen G, Huang J, Xie K, Cai J. Circadian rhythm affects the preventive role of pulsed electromagnetic fields on ovariectomy-induced osteoporosis in rats. Bone 2010; 46: 487-495.
- Akca K, Sarac E, Baysal U, Fanuscu M, Chang TL. Micro-morphologic changes around biophysically-stimulated titanium implants in ovariectomized rats. Head Face Med 2007; 3: 28.
- Khanduja KL, Syal N. Sinusoidal electromagnetic field of 50 hz helps in retaining calcium in tibias of aged rats. Indian J Exp Biol 2003; 41: 201-204.
- Liu HF, Yang L, He HC, Zhou J, Liu Y. Pulsed electromagnetic fields on postmenopausal osteoporosis in Southwest China: a randomized, active-controlled clinical trial. Bioelectromagnetics 2013; 34: 323-332.
- Kuzyk PR, Schemitsch EH. The science of electrical stimulation therapy for fracture healing. Indian J Orthop 2009; 43: 127-131.
- Turner RT, Maran A, Lotinun S, Hefferan T, Evans GL, Zhang M, Sibonga JD. Animal models for osteoporosis. Rev Endocr Metab Disord 2001; 2: 117-127.
- Shaughnessy SG, Hirsh J, Bhandari M, Muir JM, Young E. A histomorphometric evaluation of heparin-induced bone loss after discontinuation of heparin treatment in rats. Blood 1999; 93: 1231-1236.
- Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 2000; 15: 2-12.
- Saika M, Inoue D, Kido S, Matsumoto T. 17ß-estradiol stimulates expression of osteoprotegerin by a mouse stromal cell line, ST-2, via estrogen receptor-a 1. Endocrinology 2001; 142: 2205-2212.
- Hofbauer LC, Hicok KC, Chen D, Khosla S. Regulation of osteoprotegerin production by androgens and anti-androgens in human osteoblastic lineage cells. Eur J Endocrinol 2002; 147: 269-273.