Research Article - Biomedical Research (2017) Volume 28, Issue 21
Effects of puerarin on brain cell apoptosis, expression of transforming growth factor-β, vascular endothelial growth factor in hyperoxia-exposed neonatal rats
Jinghua Liu1, Kuiyi Di1, Liying Hou2 and Lei Wang1*
1Department of Neurology, the Second People's Hospital of Dongying, Dongying , Shandong Province, PR China
2Department of Joint Branch, the Second People's Hospital of Dongying, Dongying , Shandong Province, PR China
Accepted on November 16, 2017
Abstract
Aims: To investigate effects of puerarin on apoptosis of microvascular endothelial cell, expression of transforming growth factor-β and vascular endothelial growth factor in the brain of hyperoxia-exposed neonatal rats, and to explore the molecular mechanism of the protective effects of puerarin on brain.
Methods: Thirty Spraque-Dawley (SD) neonatal male rats were randomly divided into three groups: normal group, model group, and puerarin group. Each group had 10 rats. Hyperoxia brain injury model was established by exposing newborn male rats to ≥ 90% hyperoxia continuously for 14 days. Brain cell apoptosis was detected by deoxyribonucleotidyl transferase-mediated apoptosis (TUNEL). Levels of TGF-β and were measured by double antibody sandwich enzyme-linked immunosorbent assay (ELISA). Expression of TGF-β and VEGF-B protein in rat brain tissue was measured using Western blot.
Results: The apoptotic index of model group was significantly higher than that of normal group and puerarin group (p<0.05). The apoptotic index of puerarin group was significantly lower than that of model group (p<0.05). Compared with model group, TGF-β1 level was significantly decreased and VEGF-B was significantly increased in brain tissue of puerarin group (p<0.05). Western blot showed that expression level of TGF-β1 protein was significantly higher in model group than in normal group p<0.05). Puerarin down-regulated the expression level of TGF-β. Expression level of VEGF-B protein in model group and puerarin group was significantly higher than that in normal group (p<0.05), and highest expression level of VEGF-B was observed in puerarin group.
Conclusion: Puerarin may inhibit apoptosis and maintain the integrity of cerebral microvascular endothelial cells by down-regulating TGF-β expression and up-regulating VEGF expression, so as to promote angiogenesis after hyperoxia injury.
Keywords
Puerarin, Transforming growth factor beta 1, Vascular endothelial growth factor, Hyperoxia injury, Apoptosis, Angiogenesis.
Introduction
With the continuous development of neonatal intensive care, more and more low birth weight infants (ELBWI) now can survive. Due to premature birth, lungs of those infants are immature. Oxygen therapy and mechanical ventilation have become indispensable rescue measures. Hyperoxia recovery can cause neonatal brain injury, but the mechanism is still unclear. It is generally considered to be associated with brain lipid peroxidation caused by oxygen free radicals [1]. So, the development of hyperoxia brain damage protection agents has attracted more and more attention. Pueraria is the dry root of leguminous plant Pueraia lobata (Willd.) Ohwi, which is widely distributed Liaoning, Hebei, Henan, Jiangsu, Fujian and other places in China. Pueraria, also known as "South Pueraria North Senate" in China, is a kind of edible Chinese herbal medicine with high nutritional and medicinal value. Pueraria was first mentioned in "Shen Nong's Materia Medica" in the Tang Dynasty. "Ben Cao Yan Yi" in the Song Dynasty also described the medicinal value of pueraria. "Materia Medica Words" the in Ming Dynasty described that Pueraria could “relieve cold, keep skin surface clean, relieve skin heat, and relieve irritability and thirst”. Modern studies found that [2,3]. Pueraria contains flavonoids, three terpenes, coumarins and other types of compounds, among which puerarin is the main active component. Puerarin can improve immunity, enhance myocardial contractility, protect myocardial cells, reduce blood pressure and inhibit platelet aggregation [4,5]. Puerarin has been used in clinical practices to treat coronary heart disease, angina pectoris, diabetes complications, chronic pharyngitis, eye diseases, cerebral infarction, Parkinson's syndrome, sudden deafness and other diseases. Studies have shown that [6] puerarin can improve patient's neurological deficits by improving cerebral ischemia. Puerarin also has a certain role in the prevention and treatment of brain damage. However, it’s the mechanism of the function of puerarin has not been well studied. In our study, neonatal rats with hyperoxia injury were treated with puerarin, and brain cell apoptosis index, TGF-β and VEGF expression in brain tissue were measured to explore the mechanism of protective effects of puerarin on hyperoxia brain injury.
Materials and Methods
Animal model and grouping
A total of 30 male Sprague-Dawley rats were randomly divided into three groups: normal group, hyperoxia brain injury model group (model group) and puerarin treatment group (puerarin group). Each group had 10 rats. According to the methods described by Felderhoff-mueser et al. [7] and Peiser et al. [8], hyperoxia brain injury rat model was constructed. Newborn male rats were exposed to ≥ 90% hyperoxia for 14 days continuously. The rats in the puerarin group were treated with puerarin 50 mg/kg intraperitoneally after the hyperoxia exposure. Rats in model group were injected with the same amount of saline. We have added this information. Rats in model group and puerarin group were treated with routine intervention for 14 days.
Preparation of tissue specimens
After intervention for 14 days, rats in each group were treated with 10% chloral hydrate, and brain tissue was collected and fixed in 4% paraformaldehyde fixative, followed by embedding in paraffin. Hippocampus tissue was collected for in TUNEL staining, double antibody sandwich enzyme-linked immunosorbent assay (ELISA) and Western blot.
Index detection method
TUNEL staining: TUNEL kit was provided by Roche (Germany). Brown nucleus indicated positive signals. Positive cells were counted under an optical microscope (400X). The number of apoptotic cells per 100 cells was used as the apoptotic index.
ELISA assay: Brain tissue homogenate was made and ELISA was performed using TGF-β1 or VEGF-B ELISA kit (R&D, Systems) according to the manufacturer's instructions. OD values at 450 nm were measured using a microplate reader. TGF-β1 and VEGF-B concentrations were determined from the standard curve and corrected for the amount of total protein.
Western blot: Western blot was performed to detect the expression of TGF-β and VEGF-B protein in brain tissue. Total protein was extracted using cell lysate solution (Thermo Fisher Scientific, USA), and protein concentration as determined by BCA method. Then, 50 μg of protein from each sample was mix with 5x loading buffer. After denaturing, protein samples were subjected to 10% SDS-PAGE Electrophoresis, followed by transmembrane to polyvinylidene fluoride (PVDF) membrane. Blocking was performed using 5% skim milk at room temperature for 1 h. After washing, primary antibodies of TGF-β1 and VEGF-B were used to incubate with the membranes overnight at 4°C. After washing, membranes were incubated with the secondary antibody at 37°C for 2 h. ECL method was used to detect the signal. Quantity one image analysis software was used to analyze the optical density of the target band.
Statistical analysis
All statistical analyses were performed using SPSS 21.0 software. Measurement data were expressed as the means ± SD, comparisons among multiple groups were performed using variance analysis, and comparisons between groups were performed using t tests. p<0.05 was considered to be statistically significant.
Results
Comparison of apoptosis index in each group
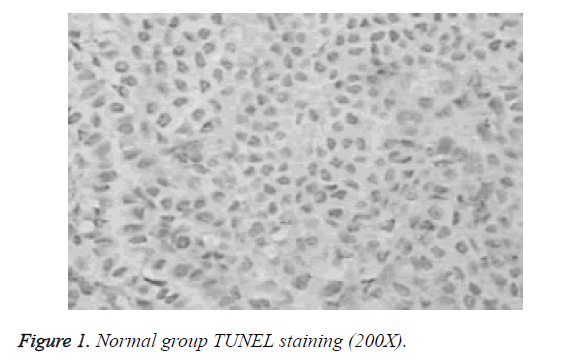
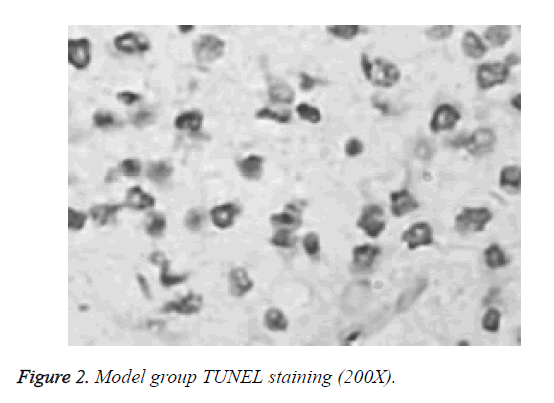
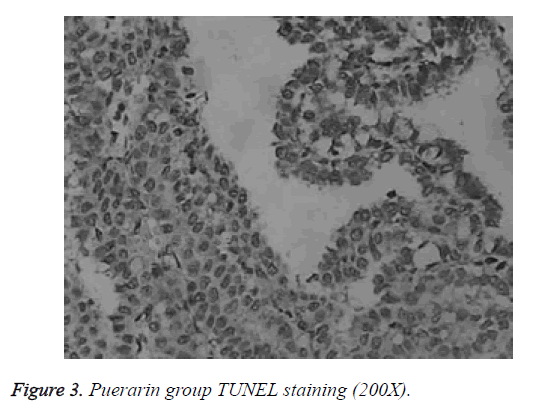
Differences in apoptotic index between normal group, model group and puerarin group were statistically significant. Apoptotic index of model group was significantly higher than that of normal group and puerarin group (p<0.05). Apoptotic index of puerarin group was significantly lower than that of model group (p<0.05) (Table 1). Apoptotic cells were mainly distributed in the cerebral cortex, hippocampus and dentate gyrus (Figures 1-3).
| Groups | N | Apoptosis index |
|---|---|---|
| Normal | 10 | 8.13 ± 1.58 |
| Model | 10 | 63.65 ± 6.22 |
| Puerarin | 10 | 19.71 ± 2.04 |
| F | 17.935 | |
| p | <0.05 |
Table 1. Comparison of the apoptosis index between different groups.
Comparison of levels of TGF-β1 and VEGF-B in brain tissue
There were significant differences in the levels of TGF-β1 and VEGF-B between normal group, model group and puerarin group (p<0.05). Levels of TGF-β1 and VEGF-B were significantly higher in model group than in normal group (p<0.05). Compared the model group, TGF-β1 concentration was significantly decreased and VEGF-B concentration was significantly increased in puerarin group (p<0.05) (Table 2).
| Groups | N | TGF-β1 (pg/ml) | VEGF-B (pg/ml) |
|---|---|---|---|
| Normal | 10 | 52.77 ± 6.02 | 128.34 ± 10.15 |
| Model | 10 | 74.63 ± 8.94 | 226.76 ± 12.28 |
| Puerarin | 10 | 66.42 ± 6.51 | 257.85 ± 11.17 |
| F | 20.092 | ||
| P | <0.05 |
Table 2. Compare the levels of TGF-β1 and VEGF-B in brain tissue.
Comparison of expression levels of TGF-β1 and VEGF-B protein in brain tissue
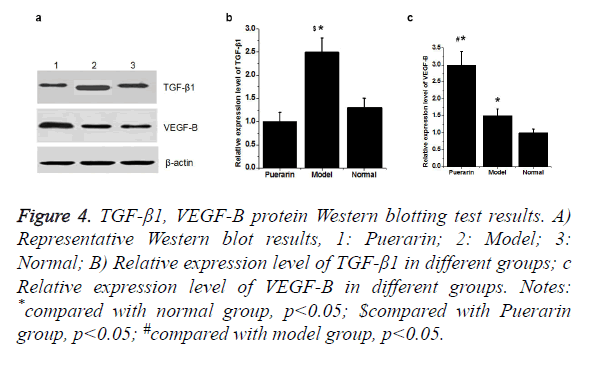
Western blot showed that the expression level of TGF-β1 protein was significantly higher in model group than in normal group (p<0.05). Compared with model group, expression level of TGF-β1 was significantly down-regulated in puerarin group. Compared with normal group, expression level of VEGF-B protein in model group and puerarin group was significantly increased (p<0.05). Expression level of VEGF-B protein in puerarin group was higher than that in model group (p<0.05) (Figure 4).
Notes: *compared with normal group, p<0.05; $compared with Puerarin group, p<0.05; #compared with model group, p<0.05.
Figure 4. TGF-β1, VEGF-B protein Western blotting test results.
A) Representative Western blot results, 1: Puerarin; 2: Model; 3: Normal;
B) Relative expression level of TGF-β1 in different groups;
C) Relative expression level of VEGF-B in different groups.
Discussion
Study of the effects of hyperoxia on neonatal development is mainly focused on lung injury and retinal damage. There are few reports on the effects of hyperoxia brain development [9]. Rodents are in rapid developmental stage of the brain within 2 weeks after birth, which is equivalent to human brain development stage of 6-month fetal to 3-year-old child, which is a sensitive period for human non-physiological oxygen toxicity. In this study, newborn rat brain was used to reflect brain development of human preterm infants, and hyperoxia brain injury model was established to explore the effects of hyperoxia on neonatal brain development and to guide clinical prevention and treatment. TUNEL staining showed that apoptotic index of the model group was all significantly higher than that of normal group and puerarin group (p<0.05). Apoptotic index of puerarin group was significantly lower than that of model group (p<0.05), indicating that hyperoxia can damage brain cells. This study found that apoptotic cells are mainly distributed in the cerebral cortex, hippocampus and dentate gyrus, suggesting the selectivity of high oxygen damage to brain cells. Therefore, inhalation time should be minimized during hyperoxia treatment to avoid oxygen damage to children.
As a multifunctional cytokine, TGF-β1 can control cell proliferation, differentiation, apoptosis and immune function through cell-surface receptor signaling pathway [10]. Studies have shown that [11,12] TGF-β can induce the expression of vascular endothelial growth factor receptor-1 (BEGFR-1) in endothelial cells to promote microvascular production, and restore ischemic blood supply. Results of this experiment show that TGF-β1 level in model group was significantly higher than that in normal group (p<0.05). Compared with the model group, TGF-β1 content was significantly reduced in brain tissue of puerarin group (p<0.05). Those results suggested that the increased level of TGF-β1 under hyperoxia injury could inhibit the growth of brain endothelial cells and induce apoptosis, while puerarin could inhibit the release of TGF-β1 in order to reduce the apoptosis of endothelial cells [13].
VEGF-B is an important member of the VEGF family. Studies have shown that VEGF-B is important in promoting myocardial vascular endothelial proliferation and angiogenesis. It has been found that [14,15], VEGF-B has the effect of stimulating neonatal neurons and promoting axonal growth. Results of our study showed that level of VEGF-B was significantly increased under hyperoxia injury. Those results suggested that hyperoxia injury could seriously stimulate the expression of VEGF-B in vascular endothelial cells. Compared with the model group, expression level of VEGF-B was significantly increased, indicating that puerarin may increase the secretion of VEGF-B, which in turn induce endothelial cell proliferation and neovascularization, so as to protect brain cells. Western blot showed that the expression level of TGF-β protein in model group was significantly higher than that in normal group (p<0.05). Compared with model group, expression level of TGF-β was down-regulated in puerarin group. Compared with normal group, expression level of VEGF-B protein in model group and puerarin group was significantly increased (p<0.05), and expression level of VEGF-B protein in puerarin group was higher than that in normal group (p<0.05). According to this, we conclude that Puerarin may inhibit the apoptosis and maintain the integrity of cerebral microvascular endothelial cells, down-regulate TGF-β expression and up-regulate VEGF expression, so as to to protect the brain cells from the damage caused by hyperoxia. However the specific mechanism remains to be further studied.
In summary, puerarin may inhibit the apoptosis and maintain the integrity of cerebral microvascular endothelial cells, down-regulate the expression level of TGF-β, up-regulate VEGF expression, and promote vascular neonatal under the conditions of hyperoxia injury, so as to protect braintissue. Puerarin is a traditional Chinese medicine extract with the advantages of low price andwide range of sources. Therefore, Puerarin may be a promising drug for the treatment of this injury. However, effects of different doses of Puerarin on brain injury were not investigated. Therefore, further studies are still needed.
References
- Zaghloul N, Nasim M, Patel H. Overexpression of extracellular superoxide dismutase has a protective role against hyperoxia-induced brain injury in neonatal mice. Febs J 2012; 279: 871-881.
- Abegaz BM, Ngadjui BT, Folefoc GN. Prenylated flavonoids, monoterpenoid furanocoumarins and other constituents from the twigs of Dorstenia elliptica (Moraceae). Phytochemistry 2004; 65: 221-226.
- Liu XY, Zhang JJ, Wang RX. Study on extracting process of pueraria flavone and evaluation of anti-oxidant activity. Food Sci 2007; 28: 232-237.
- Dong SL, Kim KM, Kim SK. Development of a method for measuring myocardial contractility with gated myocardial SPECT and arterial tonometry. J Nuclear Cardiol 1999; 6: 657-663.
- Thiem B. In vitro propagation of isoflavone-producing Pueraria lobata, (Willd.) Ohwi. Plant Sci 2003; 165: 1123-1128.
- Xu X, Zhang S, Zhang L. The Neuroprotection of puerarin against cerebral ischemia is associated with the prevention of apoptosis in rats. Planta Medica 2005; 71: 585-591.
- Dixon S J, Stockwell B R. The role of iron and reactive oxygen species in cell death. Nature Chem Biol 2014; 10: 9-17.
- Peiser C, Felderhoff-mueser U, Koehne P. Hyperoxia decreases, injury caused by phorbolester increases VEGF expression in the brain of newborn rats. Pediatric Res 2005; 58: 404.
- Özdemir ÖM, Gözkeser E, Bir F. The effects of resveratrol on hyperoxia-induced lung injury in neonatal rats. Pediatrics Neonatol 2014; 55: 352-357.
- Lin LL, Huang HC, Juan HF. Discovery of biomarkers for gastric cancer: A proteomics approach. J Proteomics 2012; 75: 3081-3097.
- Zhou Y, Yang P, Li A. Prostaglandin E2 reduces swine myocardial ischemia reperfusion injury via increased endothelial nitric oxide synthase and vascular endothelial growth factor expression levels. Biomed Rep 2017; 6: 188-194.
- Melly LF, Marsano A, Frobert A. Controlled angiogenesis in the heart by cell-based expression of specific vascular endothelial growth factor levels. Hum Gene Ther Methods 2012; 23: 346-356.
- Szymanska J, Goralczyk K, Klawe JJ. Phototherapy with low-level laser influences the proliferation of endothelial cells and vascular endothelial growth factor and transforming growth factor-beta secretion. J Physiol Pharmacol 2013; 64: 387-391.
- Becker J. Inhibition of neuroblastoma progression by targeting lymphangiogenesis: Role of an endogenous soluble splice-variant of VEGFR-2. Tumors of the Central Nervous System. Springer, Netherlands, 2014.
- Zhang J, Ye J, Ma D. Cross-talk between leukemic and endothelial cells promotes angiogenesis by VEGF activation of the Notch/Dll4 pathway. Carcinogenesis 2013; 34: 667-677.