Review Article - Journal of Clinical and Experimental Toxicology (2018) Volume 2, Issue 2
Effects of Pseudocedrela kotschyi stem bark on blood chemistry and histology of some organs in rats.
Attah D. Daniel*
Department of Biological Sciences, Zoology unit, Kebbi State University of Science and Technology, Aliero, Nigeria
- *Corresponding Author:
- Attah, D. Daniel
Department of Biological Sciences
Kebbi State University of Science and Technology
Nigeria
Tel: +2348038350460
E-mail: dd.attah@yahoo.com
Accepted date: July 05, 2018
Citation: Daniel A. Effects of Pseudocedrela kotschyi stem bark on blood chemistry and histology of some organs in rats. J Clin Exp Tox. 2018;2(1):25-31.
Abstract
Pseudocedrela kotschyi is a plant commonly found in Africa and is used by traditional practitioners in the treatment of malaria, as a purgative, antipyretic, anti-ulcer and wound dressing agent. The objective of this study was to investigate the acute and sub-chronic toxicity of the stem bark of the plant in Wistar rats. In acute test, the limit test dose of 1,500 mg/kg was orally administered to Wistar rats and then observed individual for 72 hours post dosing and once every day for 14 days, and the Median lethal dose (LD50) was found to be 1,225 mg/kg body weight. The subchronic toxicity study was done using standard protocol in graded doses of 100 mg/kg, 200 mg/ kg, and 400 mg/kg of the plant extract for 28 days. At the end of treatment, chloroform was used to euthanize the rats and was examined both grossly and histopathologically. Administration of extracts for the 28 days at various doses did not record any death of the rats; however, loss of appetite and reduced activity were observed in rats dosed with 400 mg/kg. Mild histological lesions were also observed in the liver, kidney and heart respectively. It is suggestive that the extract has a wide safety margin, meanwhile, the histopathological lesions found in the various organs suggests that the extract should be administered with caution when used for prolonged period.
Keywords
Pseudocedrela kotschyi, Wistar rats, histopathology
Introduction
All modern medicines are derived originally from ancient herbal tradition and about 80% of the population in the developing world still relies on traditional medicine [1]. Plants produce bioactive compounds that act as defense mechanism against predators and at the same time may be toxic in nature for our health. With the increased interest in the pharmacological activities of these medicinal plants, there is a surge for thorough scientific investigations of these medicinal plants for medicinal purposes as safe, non-toxic and pharmacologically active.
Pseudocedrela kotschyi commonly called dry-zone cedar belongs to the family Meliaceae which grows in the savannah zone in tropical Africa from Nigeria, Senegal east to western Ethiopia and Uganda. The bark decoction or macerations of the plant are applied externally to ulcers, sores, rheumatism, leprosy and itches. Internally they are used to treat fever, stomachache, diarrhea and dysentery [2], and it is reported to possess anthelmintic effects on Ascaris suum [3]. It is commonly used for oral hygiene in the tropics and found effective against Staphylococcus areus and Staphylocuccus auriculus [4]. In other studies, a significant difference (p<0.05) in reducing parasitaemia at 39.43% in Plasmodium infected albino rats treated with ethanol extract of P. kotschyi was reported [5].
The plant is reported to contain limonoids, including pseudrelones, dichloromethane [6]. There is other report of toxicity of P. kotschyi and other members of its genera in literature [7,8]. While the stem bark of the plant is widely used medically, little studies have been reported on its toxicity. Determination of the toxicity profile of P. kotschyi will provide a guide on its oral application for medicinal proposes. There is, therefore, the need for a thorough safety assessment of the stem bark of the plant. This study is aimed at evaluating the possible toxicity via biochemical and haematological assessment and selected histological effects of aqueous extracts of P. kotschyi stem bark on Wistar rats.
Materials and Methods
Plant materials and preparation of extract
Pseudocedrela kotschyi plant was collected on 18th December, 2015 at Ribah in Danko/Wasagu Local Government Area of Kebbi State Nigeria and was identified by a botanist in the Department of Biological Sciences, Kebbi State University of Science and Technology, Aliero, Nigeria. The plant materials (stem bark) were washed with clean water, shade –dried for seven days and ground to obtain fine power. Decoction method of extraction was used; 400 g of the dried fine powder was weighed into an empty clean beaker and 1 liter of distilled water added and heated at 90°C for 4 hrs and allowed to stand for 4 days. After this duration, it was shaken for 20 minutes and filtered into a clean beaker using size 1mm Whatman filter paper. The volume of the filtrate was noted and placed in an electric drier and evaporate slowly at 45°C [9].
Experimental animals
Adult Wistar rats were obtained at animal house, Usmanu Danfodiyo University, Sokoto, Nigeria. Twenty (20) healthy rats weighing between 90-200 g were housed separately under standard animal house conditions (temperature: 23 ± 2°C; photoperiod: 12 h light and 12 h dark; humidity: 45-50%) for 2 weeks to allow for acclimatization. They were fed with standard diet (Vital feed, Nigeria Limited), and clean water ad libitum. The animals were maintained according to the National Institute of Health Guide for the care and use of laboratory animals [10], and according to the experimental protocol approved by the animal ethical committee of the Department of Biological Sciences, Kebbi State University of Science and Technology, Aliero, Nigeria.
Preliminary phytochemical screening
Preliminary qualitative phytochemical screenings were conducted on the aqueous extract of the plant using standard methods [11,12].
Acute toxicity study
Acute oral toxicity study was conducted according the guidelines of Organization of Economic Co-operation and Development No. 407 [13]. This test was conducted in two phases according to the method of Lorke D. 1983, for the evaluation of the safety of herbal medicines [14]. In phase I, nine Wistar rats were weighed and randomly divided into three treatment groups (A, B and C) of three animals each. The animals were deprived of food for 16-18 hrs prior to administration of the plant extract. The plant extract was administered orally in geometrically increasing doses: Group A for low doses of 500 mg/kg, group B for medium dose of 2500 mg/kg and group C, for high dose of 5000 mg/kg and observed for signs of toxicity immediately and up to 72 hrs after administration.
In phase II, three groups of one rats (n=1) were also deprived of food for 16-18 hrs prior to administration of the plant extract. The rats were the given P. kotschyi extracts orally in geometrically increasing doses: Group A 6000 mg/kg, Group B 8000 mg/kg and Group C 10,000 mg/kg. Observation of signs of toxicity such as tremors, convulsions, salivation, weakness or aggressiveness, food refusal, noisy breathing and mortality were observed systematically and recorded with individual records being maintained for each group in each phase. The median lethal dose (LD50) was thus calculated as the geometric mean of the least dose that kills a rat and the highest dose that doesn’t kill any rat.
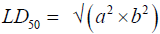
Where: a=least dose that caused toxicity signs, while
b=highest dose that did not produce signs of toxicity.
Sub-chronic toxicity study
Organization for Economic Cooperation and Development No. 407 test guidelines which described short-term repeat-dose toxicity testing was used for the study [15]. Twenty (20) Wistar rats were weighed and grouped randomly into four groups (A, B, C and D) of five rats each. The animals in group A served as the control and were administered with distilled water. Group B was administered with 100 mg/kg, group C 200 mg/kg and group C 400 mg/kg of the plant extract respectively. They were given food 30 minutes after administration of the extracts by gavage and subsequently fed with standard pelletized commercial grower ration (Vital feed, Nigeria Limited). Drinking water was provided ad libitum. Administration of the extract and body weights of the animals were done daily for 28 days. The animals were observed daily to detect differences in appearance, decoloured fur, diarrhoea, bloody stool and constipation, loss of appetite and thirst, and lack of interest in the environment.
Biochemical analysis
On the 29th day, after an overnight fast, the rats were anaesthetized with chloroform and blood samples for haematological and biochemical analyses were collected into tubes with and without EDTA respectively. White blood cell (WBC) count, red blood cells (RBC) count, haemoglobin (Hb) levels, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) were determined using BC 2300 Haematology Analyzer (Shezhen Mindray Biomedical Electronics Co., Ltd, China). Biochemical analysis was performed after allowing the blood sample to clot for 30 minutes and then centrifuge at 3000 rpm for 10 minutes. The serum was separated and analyzed for some enzymes; alanine aminotransferase (ALT), aspartete aminotransferase (AST), ALP, creatinine, alkaline phosphatase, total protein and urea using the Humalyzer 2000, German model [16].
Histological analysis
Three animals from each group were randomly selected and the heart, liver and kidney were carefully harvested out, washed with normal buffered saline, weighed to determine the absolute and relative organ weights respectively. The organs were then fixed in 10% buffered formalin for 24 hrs before being trimmed and then further processed by tissue processing method [17]. Histology of the tissues were performed by preparing, 6-8 μm sections of the tissues with the help of Microtome (Leica, RM 2145). These sections were then deparaffinated in xylene, passed through 80% to 100% alcohol, and stained with hematoxylin and eosin (H&E) for assessment of the organs [18]. The slides prepared by this process were observed under microscope for the study of the organ cells [19]. These slides were photographed through Nikon Advanced Research Microscope OPTIPHOT Model X2T-21E equipped with Nikon Microphotography system; Model UFXDX- 35 and phase contrast N plan. The absolute organ weights were determines and the relative organ weights (ROW) were calculated for each experimental animal for each group [20].
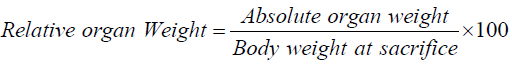
Statistical analysis
Data are expressed as mean ± standard error of mean (SEM). Statistical analysis was performed by one-way analysis of variance (ANOVA) with Turkey test to evaluate significant differences between groups. Values of p<0.05 were considered significant. All statistical analysis was carried out using the SPSS version 20.0.
Results
Phytochemical analysis
Result of the phytochemical screening of the aqueous extract of P. kotschyi revealed the presence of flavonoids, saponins, cardiac glycosides, anthraquinones, tannins, alkaloids, triterpenoids and resins (Table 1).
| Phytochemical Constituents | Inferences |
|---|---|
| Tannins | +++ |
| Anthraquinones | ++ |
| Saponin | ++ |
| Triterpenoids | + |
| Glycosides | + |
| Flavonoids | ++ |
| Alkaloids | + |
| Resins | + |
Table 1. Phytochemical constituents of the aqueous extract of P.kotschyi.
Acute toxicity study
In oral acute toxicity studies, no animal death occurred in phase I, however, death occurred in the groups treated with 5,000 mg/ kg and 10,000 mg/kg in phase II. However, lack of interest in the environment and reduce activity was observed in the group treated with 2,000 mg/kg.
Sub-chronic toxicity study
In the sub-chronic toxicity studies no death were observed at the end of the treatment. However, loss of appetite, cough and reduced activity were observed in the group treated with 400 mg/kg. Similarly, there was a significant difference (p<0.05) in body weight between the group treated with 400 mg/kg of the extract and the control (Table 2)
| Concentration | Mean body weight (mg/kg) | ||||
|---|---|---|---|---|---|
| Post-administration duration (days) | |||||
| 0 | 7 | 14 | 21 | 28 | |
| Control | 256.9 ± 2.1a | 258.9 ± 0.6a | 260.4 ± 0.2a | 269.4 ± 0.3a | 272.7 ± 0.7a |
| 100 mg/kg | 253.7 ± 0.9a | 254.1 ± 3.7a | 258.1 ± 9.6a | 247.3 ± 22.3a | 247.1 ± 21.2a |
| 200 mg/kg | 179.9 ± 25.5c | 178.9 ± 22.3b | 179.3 ± 1.1ab | 177.1 ± 0.5ab | 175.5 ± 1.5a |
| 400 mg/kg | 222.6 ± 18.5b | 220.5 ± 18.1a | 187.0 ± 2.2a | 158.9 ± 1.5a | 167.2 ± 0.6a |
Table 2. The effect of P. kotschyi aqueous extract on mean body weight (kg) changes in the treated and control rats for 28 days.
Table 3 shows the haematological parameters for the rats after sub-chronic toxicity for 28 days. The packed cell volume (PCV), haemoglobin (Hb) and red blood cell (RBC) of animals treated with 400 mg/kg were significantly different (p<0.05) when compared with the control. White blood cell (WBC) values of the treated animals showed no significant difference (p<0.05) with the control. However, rats dosed with 200 mg/kg and 400 mg/kg treated groups showed decreased WBC counts compared with the control. The result of the haematological indices (MCV, MCH and MCHC) of the groups treated with 100 mg/kg and 200 mg/kg did not differ significantly with the control. However, the group dosed with 400 mg/kg recorded a significant (p>0.05) decrease in the values.
| Parameter | Concentrations (mg/kg) | |||
|---|---|---|---|---|
| Control | 100 | 200 | 400 | |
| PCV (%) | 8.4 ± 0.6b | 8.4 ± 0.3ab | 7.9 ± 0.4ab | 5.9 ± 0.3a |
| Hb (g/dl) | 3.1 ± 0.3a | 3.1 ± 0.3a | 2.6 ± 0.5a | 2.4 ± 0.3a |
| WBC (x103 mm3) | 3.8 ± 0.3a | 3.7 ± 0.2ab | 3.6 ± 0.3ab | 2.8 ± 0.3a |
| RBC (x106 mm) | 1.1 ± 0.2a | 1.1 ± 0.1a | 1.0 ± 0.1a | 0.6 ± 0.2a |
| MCV | 78.9 ± 3.3a | 79.5 ± 4.3a | 79.4 ± 4.8b | 96.1 ± 2.9b |
| MCH | 29.1 ± 1.1a | 29.2 ± 2.4a | 26.8 ± 1.1ab | 39.1 ± 2.3b |
| MCHC | 36.8 ± 1.1a | 36.7 ± 1.1a | 33.7 ± 2.2a | 40.7 ± 2.8b |
Table 3. Mean haematological parameters for rats after 28 days treatment with the aqueous of P. kotschyi.
The effect of the plant extract on some liver marker enzymes in rats recorded a significant (p>0.05) increase in serum transaminases (AST and ALT) activities. There were no significant difference (p<0.05) in serum ALP activity between the animals treated with the plant extract and control, but decrease of the values were recorded in treated animals when compared with the control (Table 4).
| Parameter | Extract Concentrations (mg/kg) | |||
|---|---|---|---|---|
| Control | 100 | 200 | 400 | |
| AST (U/ L) | 3.9 ± 0.4a | 4.4 ± 0.6a | 5.3 ± 0.3ab | 6.5 ± 0.3b |
| ALT (U/L) | 3.2 ± 0.5a | 3.7 ± 0.3a | 4.1 ± 0.2ab | 4.9 ± 0.3b |
| ALP (U/L) | 22.8 ± 0.6a | 25.2 ± 0.4b | 29.6 ± 0.3c | 33.6 ± 0.9d |
| Total Protein (g/dl) | 1.4 ± 0.31a | 1.2 ± 0.26a | 1.2 ± 0.1a | 1.1 ± 0.1a |
| Glucose (mg/dl) | 22.0 ± 0.6c | 19.6 ± 0.7b | 18.6 ± 0.7b | 14.9 ± 0.5a |
| Creatinine (mg/dl) | 0.5 ± 0.1a | 0.3 ± 0.1a | 0.3 ± 0.3a | 0.3 ± 0.1a |
| Urea (mmol/l) | 4.6 ± 0.1c | 3.8 ± 0.1b | 3.3 ± 0.1a | 3.1 ± 0.1ab |
Table 4. Effect of aqueous extract of P. kotschyi on serum proteins and liver function of treated and control rats for 28 days.
The mean effects of oral administration of P. kotschyi stem bark on some biochemical indices in rats are shown in Table 4. Total proteins and glucose recorded lower levels for treated rats compared with the control. In the same manner, treated animals (200 and 400 mg/kg) showed significant (p>0.05) reduction in values of urea compared with the control. There was no significant different between treated animals and control on creatinine values, but decrease values of the parameter were recorded in treated animals compared with the control.
Histopathological analysis
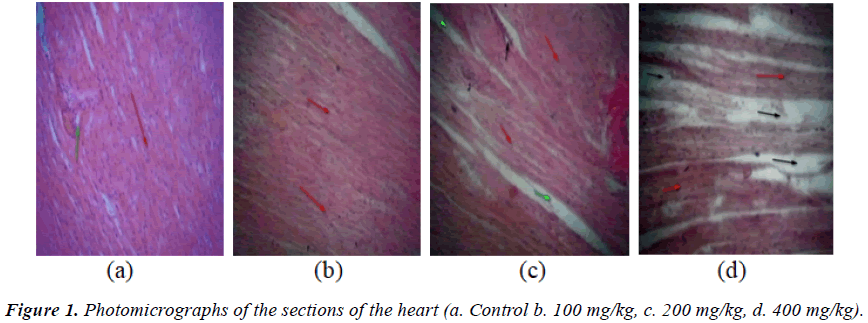
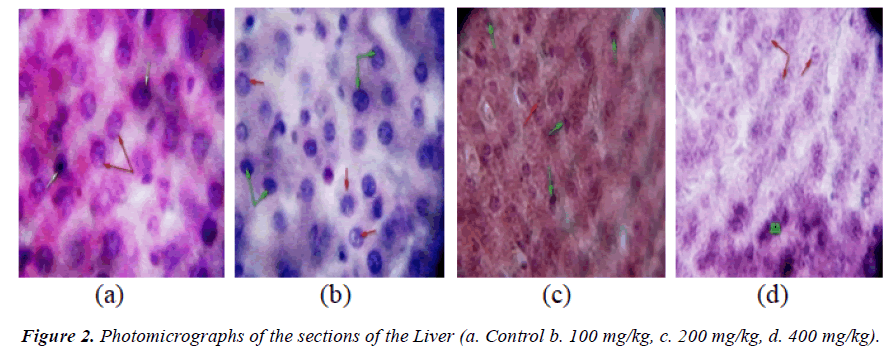
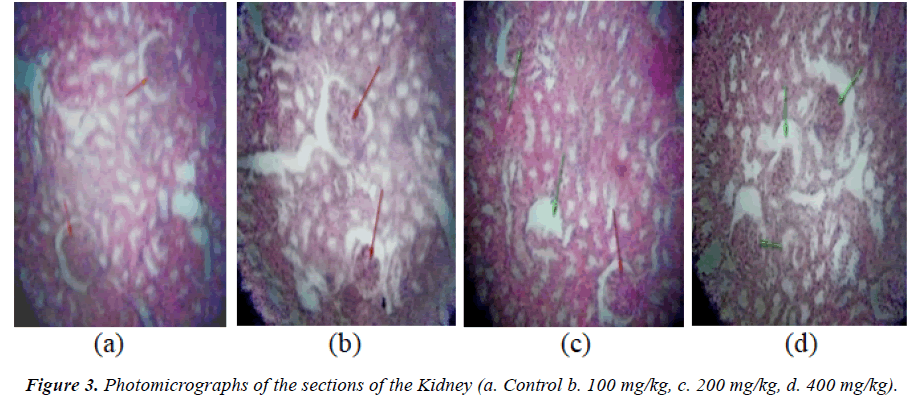
Result of the relative organ weights of some organs in control and treated animals with the sub-chronic doses of the aqueous extract of P. kotschyi recorded a significant raise in the values of the parameters in the groups treated with 200 and 400 mg/kg compared with the control (Table 5). Macroscopic examination of the vital organs (the liver, kidney and heart) of experimental animals showed noticeable differences between the control and test groups (Figures 1-3).
| Parameter (%) | |||||
|---|---|---|---|---|---|
| Concentration | Heart | Liver | Kidney | Lung | Spleen |
| Control | 0.01 ± 0.0a | 0.6 ± 1.1a | 0.1 ± 0.0a | 0.1 ± 0.0a | 0.1 ± 0.0a |
| 100 mg/kg | 0.01 ± 0.0a | 0.6 ± 0.0ab | 0.1 ± 0.0a | 0.2 ± 0.0a | 0.1 ± 0.0a |
| 200 mg/kg | 0.1 ± 0.0a | 0.8 ± 0.0ab | 0. 1 ± 0.0a | 0.2 ± 0.0a | 0.1 ± 0.0a |
| 400 mg/kg | 0.1 ± 0.0a | 0.8 ± 0.0b | 0.2 ± 0.0b | 0.2 ± 0.0a | 0.1 ± 0.0a |
Table 5. Mean effect on relative organ weight of control and treated rats with P. kotschyi extracts for 28 days.
In the group treated with 100 mg/kg, the photomicrographs of the organs (the liver, kidney, and heart) did not reveal varying morphological changes when compared with the control (Figures 1a and 1b, 2a and 2b, 3a and 3b). However, the glomerulus of the kidney was seen with slight degenerated changes and congested collecting ducts.
Varying changes in the histopathological examination of the animals treated with 200 mg/kg of the plant extract, revealed increased interseptal space muscular orientation of the heart’s cardiac muscles (Figure 1c) compared with the control, while the liver revealed necrotized hepatocytes and abundant macrophages (Kuffa cells) (Figure 2c). The photomicrograph of the kidney of this group also showed degenerated atrophy glomerulus with congested collecting ducts (Figure 3c) when compared with the control group.
Histological examination of the heart of animals treated with 400 mg/kg, showed degenerated muscular orientation of the cardiac muscles (Figure 1d), while the liver showed clear zone of necrotized cells (Figure 2d), and the kidney revealed degenerated atropy glomerulus with normal collecting ducts (Figure 3d) when compared with the control respectively.
Discussion
Phytochemical screening of the aqueous extract of P. kotschyi revealed the presence of tannins, anthraquinones, triterpenoids, glycosides, saponins, flavonoids, alkaloids and resins. This agreed with Otimenyin et al. and Alhassan et al. , however, resins were not detected by Alhassan et al. and Otimenyin et al. respectively [21,22]. This variation in plant secondary metabolites can be as a result of both genetic and environmental factors [23]. The geographical location of the sites of sample collection could also be the chief factor responsible for variation in the chemical constituents of the samples in the two studies. Additionally, the season of the year could also play a role on the type and quality of chemical constituents found in a particular plant sample [24]. Samples were collected at the end of the rainy season in December in Bedi Kebbi State in the present study; whereas Alhassan et al. collected their samples in June around Nasarawa in Nasarawa State and Otimenyin et al. collected their samples in July in Calabar Nigeria [21,22].
The oral acute toxicity of the stem bark of P. kotschyi in rats was estimated to be 1,225 mg/kg. The toxicity scale of Hodge and Sterner, classifies any compound with an oral LD50 of ≤ 1 mg/kg to be highly toxic, an LD50 between 1-50 mg/kg to be highly toxic, an LD50 between 50-500 mg/kg as moderately toxic, slightly toxic if the LD50 is between 500-5000 mg/kg, and practically non-toxic if it is between 5000-15,000 mg/ kg [25]. Therefore, the extract can be said to be slightly toxic orally. This result agrees with other findings Ojewale et al. who recorded an LD50 of 15 g/kg BW in rats treated with P. kotschyi extract at an oral dose of 20.0 g/kg bw, and the finding of Akuodor et al. [4,7], that also reported an LD50 of 775 mg/kg intra peritoneal in mice with P. Kotschyi extract at doses 10 mg/kg, 100 mg/kg and 1000 mg/kg body weight.
The loss of weight observed in treated animals in this study may be due to loss of appetite observed in the experimental rats. The reduction in weight observed in treated animals may also be as a result of the presence of phenolic and tannin phytoconstituents screened in the plant. These constituents have been reported to interfere with the absorption of nutrients [26,27], consequently the weight of the treated animals will certainly reduce because the food taken will not be fully utilized by the body.
The presence of a significant (p<0.05) difference in blood parameters (PCV, RBC, WBC, MCV, MCH and MCHC) of animals treated with both the acute and sub-chronic doses of P. kotschyi, suggests that the plant fraction may have some adverse effect on the blood, especially with the highest concentrations (400 mg/kg) tested in this study. The significant changes in serum electrolytes further confirm this suspicion. This is because saponins which have been reported to have deleterious haemolysing effect on circulating erythrocytes [12] were detected in the plant. This finding suggests that the plant may be able to cause anaemia at higher doses or if used for a long time. Plants with saponins have been reported to cause anaemia by having adverse effect on the haematological parameters.
Liver and kidney plays significant role in metabolic processes. While the liver detoxifies substances that are harmful to the body, the kidney helps in maintenance of homeostasis by reabsorbing vital substances and excretion of waste products [28]. In assessing liver damage by hepatotoxins, the determination of enzyme levels such as ALT and AST is largely used [29]. Serum ALP on the other hand, is related to the function of hepatic cells and increased levels is due to increase synthesis of the enzyme in the presence of increasing biliary process [30]. Albumin is synthesized in the liver, and increased levels are implicated in malfunction in hepatic synthetic ability while excessive levels are usually due to dehydration [31]. Thus, the elevated levels of AST, ALT and ALP observed in this study may be an indicative of liver dysfunction as a result of prolonged administration of the plant in this study. This is further confirmed by the presence of hepatocytes with clear zone of narcotized cells as revealed by the histological micrograph of the liver of rats treated with 400 mg/kg (Figure 2d) and the presence of degenerated atrophy glomerulus of kidney (Figure 3d).
Urea is the first acute renal marker and increases only when the majority of renal function is lost [32]. The present result revealed a reduction, though not statistically significant, in the levels of urea and creatinine both in the acute and sub-chronic oral doses of the plant extract compared to the controls. This suggested that the plant is slightly nephrotoxicant because Abdel-Moneim & Ghafeer [33] reported that nephrotoxicants such as cadmium increases serum level of urea and Creatinine in rats whiles nephroprotective agents such as honey reduces serum urea and Creatinine levels. The significant changes observed in histology of the kidney and liver in this study may be due to the presence of anthraquinones in the extract which have been reported to produce free radicals and hydrogen peroxide thought to damage cells of the body [8].
The administration of the sub-chronic oral doses of the plant resulted in reduction of glucose levels in a dose dependent manner with highest dose having a significant reduction (p<0.05). Glucose is one of the clinically important carbohydrates. Disorders of carbohydrate metabolism such as diabetes are evaluated in part by measurement of plasma glucose in either the fasting state or after suppression or stimulation [34]. The significant reduction in glucose level in the test group (400 mg/kg) implies that P. kotschyi may serve as a good source for antidiabetic agent. This finding is in line with the report of Ojewale et al., who recorded a significant dose dependent reduction in glucose levels after administration of ethanolic roots extract of P. kotschyi in treated diabetic rats [7]. In addition, any parasitic infection that largely depends on glucose for survival like in Trypanosomiasis can be deprived of this important nutrient [34], and therefore may also serve as source of treatment for the host.
The high amount of macrophages was observed from the photomicrograph of the kidney of rats treated with 200 mg/kg of the plant extract (Plate 2b), which means that P. kotschyi might have possessed the ability to stimulate the immune reaction through the production of antibodies or might have influenced both humoral as well as cell mediated immune system. Therefore the extract of this plant may be useful in infections where the immune system is compromise.
Conclusion
The pharmacological investigation for oral toxicity properties of the stem bark of P. kotschyi in Wister rats is mild. However, the extract may have deleterious effects on the liver and kidney at high doses on prolonged administration. This findings support its safe ethnomedicinal use at moderate doses and it should not be used for a long period.
Acknowledgements
The author is grateful the Tertiary Trust Fund (TETFUND) Nigeria, for funding this research work. I am also grateful to the Department of Biological sciences, Kebbi State University of Science and Technology, Aliero, Nigeria, for granting me the Laboratory facilities and staff assistance.
References
- Ravindra GM, Anita AM. A review on Anthelmintic plants. Natural product Radiance 2007;7:466-75.
- Arbonnier M. Trees, shrubs and lianas of West African dry zones. CIRAD, Margraf Publishers Gmbh, MNHN, Paris, France 2004; pp:573.
- Emaruk E, Olila D. Ascaridal Activity of some Medicinal Plants Used by Then Karimojong: A nomadic Pastoral Community in Uganda. Journal of Animal and Veterinary Advances 2006;5:724-8.
- Akande JA, Hayash Y. Potency of extract contents from selected tropical chewingsticks against Staphylococcus areus and Staphylococcus aurculus. World Journal of Microbiology and Biotechnology 1998;14:235-8.
- Dawet A, Yakubu DP, Wannang NN, et al. In vivo Antimalarial Activity of Stem Bark of Dry Zone Cedara Pseudocedrela kotschyi (Meliaceae) in Mice. European Journal of Medicinal Plants 2014;4:342-52.
- SEPASAL. Pseudocedrela kotschyi. In: Survey of Economic Plants for Arid and Semi-Arid Lands (SEPASAL) database. Royal Botanic Gardens, Kew, Richmond, United Kingdom 2007.
- Ojewale AO, Olaniyan OT, Faduyile FA, et al. Testicular Protective Effects of Ethanolic Roots Extract of Pseudocedrela kotschyi on Alloxan induced Testicular Damager in Diabetic Rats. British Journal of Medicine Research 2014;4:548-63.
- Akuodor GC, Essien AD, Essiet GA, et al. Evaluation of Antipyretic potential of Pseudocedrela kotschyi Schweint. Harms (Meliaceae). European Journal Medical Plants 2013;3:105-13.
- Muyibi SA, Olorode BR, Onyeyili PA, et al. Haematological and Histopathological changes of Senna occidentalis leaf extract in rats. Nigerian Journal of Natural Products and Medicine 2000;4:48-52.
- NIH. NIH Guide for the care and use of laboratory Animals. NIH Publication 1996; pp:23-83.
- Trease GA, Evans WC. Textbook of Pharmacognosy, 13th edn. Bailliere, London 2002.
- Sofowora A. Phytochemical screening of traditional medicine in Africa. 2nd Edition, Spectrum Books Limited, Nigeria 1993; pp:150-6.
- OECD (testing guideline, 401). Guidelines for thew testing of Chemicals. OECD 401. Acute oral toxicity. Paris: Organization for Economic and development 1991.
- Lorke D. A new approach to practical acute toxicity testing. Archives of Toxicology 1989;53:275-89.
- OECD (testing guideline, 407). Repeated Dose 28-days oral toxicity study in Rodents; In-Guidance document for the development of OECD guideline for testing of chemicals. Environmental monogr 1995; pp:76.
- Konan NA, Bacchi EM, Loncopan N. Acute, subacute toxicity and genotoxic effect of ahydroethanolic extract of the cashew (Anacardium occidentale L). Journal of Ethnopharmacology 2007;110:30-8.
- Thanabhorn S, Jaijoy K, Thamaree S. Acute and subacute toxicity study of the ethanol extract Fromlonicera japonica thunb. Journal of Ethnopharmacol 2006;107:370-3.
- Feres CA, Madalosso RC, Rocha OA. Acute and chronic toxicological studies of Dimorphandra mollis in experimental animals. Journal of Ethnopharmacology 2006;108:450-6.
- Curran RC. Colour atlas of histopathology. Harvey Miller publishers. Oxford University Press 1990; pp:30-107.
- Swain SR, Sinhai BN, Murthy PN. Sub-chronic Toxicity of the Hydrochloric extracts of Rungia pectinata leaves. Pharmacologyonline 2008;2:461-6.
- Otimenyin SO, Uguru MO, Atang BL. Ant-inflammatiroy and Analgesic Activities of Ficus thonningii and Pseudocedrela kotschyi extracts. Nigerian Journal of Pharmaceutical Resaerch 2004;3:82-5.
- Alhassan MA, Ibrahim M, Musa IA. Phytochemical screening and antimicrobial evaluation of stem bark of Pseudcedrela kotschyi (Schweinf.) Herms. British Journal of pharmaceutical Research 2014;4:1934-44.
- Koupai-Abyazani MR, Muir AD, Bohm BA, et al. Developmental changes in the composition of Proanthoicyanidins from leaves of sainfion (Onobrychis vicifolia Acop) as determined by HPLC analysis. Journal of Agric and Food Chemistry 1993;41:1066-70.
- Marais JP, Mueller HI, Brandt EV, et al.Polyphenols, condensed tannins and other natural products in Onobrychis vicifolia (Sainfion). Journal of Agric and Food Chemistry 2000;48:3440-7.
- CCHOS. What makes chemicals poisonous? 1999; pp:417.
- Anthanasiadou S, Kyriazakis I. Plant secondary metabolites: antiparasitic effects and their role in ruminant production system. Proceedings of the Nutrition Society 2004;63:631-9.
- Kofi D, Laud NKO, Wonder KMA, et al. Acute and Sub-Chronic Toxicity Studies of Aqueous Extracts of Root Bark of Cassia sieberiana D.C in Rodents. Journal of Applied Pharmaceutical Science 2014;4:84-9.
- Greaves P. Histopathology of preclinical toxicity studies: interpretation and Relevance in Drug Safety Evaluation. New York Academic Press 2007; pp:149.
- Dobbs NA, Twelve CJ, Gregory W, et al. Epirubicin in patients with liver dysfunction development and evaluation of a novel dose modification scheme. European Journal of Cancer 2003;39:580-6.
- Moss DW, Butterworth PJ. Enzymology and Medicine. London pitman Medical 1974; pp:345.
- Pritchett S. Abnormal LFTs. Innovait: The RCGP. Journal for Associates in Training 2009;2:140-7.
- Borges LP, Moro AV, Nogueira CW, et al. Protective effect of Diphenyl diselenide on acute liver damage induced by 2-nitropropane in rats. Toxicology 2005;210:1-8.
- Abdel-Moneim WM, Ghafeer HH. The potential protective effect of Natural honey against Cadmium-induced hepatotoxicity and nephrotocicity. Mansoura Journal of Forensic Medicine ans Clinical Toxicology 2007;15:75-97.
- Abubakar A, Ogbadoyi EO, Okogun JI, et al. Acute and Sub-chronic Toxicity of Tridax procumbens in Experimental Animals. Journal of Experimental Science, Toxicology and Technology 2012;1:19-27.