Research Article - Journal of Aging and Geriatric Psychiatry (2018) Journal of Aging and Geriatric Psychiatry (Special Issue 1-2018)
Effects of periodical multicomponent exercise training and detraining for 18 months on physical function in older adults with dementia.
Seung Youn Hong*Division of Silver Industry, Kangnam University, Seoul, Republic of Korea
- *Corresponding Author:
- Seung-youn Hong Ph.D., MEd
Division of Silver Industry
Kangnam University
Seoul
Republic of Korea
Tel: 82-31-280-3975
Fax: 82-31-899-7211
E-mail: yoni91@hotmail.com
Accepted Date: February 12, 2018
Citation: Hong SY. Effects of periodical multicomponent exercise training and detraining for 18 months on physical function in older adults with dementia. J Aging Geriatr Psychiatry. 2018;2(1):10-14
Abstract
Purpose: Verify the effects of periodical multicomponent exercise training and detraining on the physical function in older adults with dementia. Method: Seventeen older adults with dementia (mean age 72, MMSE 17.8) performed multicomponent exercise twice a week for a total of 18 months. The assessment was made six times: 1) at the baseline, 2) 24 weeks of training, 3) 12 weeks of first detraining, 4) 12 weeks of retraining, and 5) 12 weeks of second retraining after second 12 weeks of detraining. Sit to stand, arm curl, 8 feet Time to up and go, 4-meter walk test, Short Physical Performance Battery were assessed. Nonparametric Friedman's test and Wilcoxon matched pairs signed rank test was conducted with an alpha-level of 0.05. Results: Significantly training-related improvements for the sit to stand, arm curl, 8-foot TUG, 4-meter walk, and SPPB. Following two times of 12 week-detraining and retraining, significantly enhanced performance (compared to baseline) was still presented in sit to stand and insignificantly maintained performance (compared to baseline) was presented in arm curl, 8-foot TUG, 4-meter walk and SPPB. Conclusion: The present study tested and confirms that the effect of periodical multicomponent exercise training and detraining improved or maintained physical function in older adults with dementia.
Keywords
Exercise, Dementia, Function, Detraining, Retraining
Introduction
As the size and proportion of the population as 65 and older continue to increase, the number of Korean with dementia will grow. The number of aging people will escalate rapidly in 2020, as the baby boom generation (born in 1953) will begin to reach age 65 and beyond. An estimated 0.64 million Korean of people age 65 and older have dementia in 2015. This number indicates that one in ten people age 65 and older have some dementia and one-third of people age 85 and older (38.4%) have dementia have dementia as per the report of National Institute of Dementia in 2017.
As a result, the medical and long-term care expenses of dementia patients are rising year by year. Government expenses for dementia were 0.9% of GDP in 2015 and are predicted to increase in 3.8% of GPD in 2050 as per the report of National Institute of Dementia in 2017.
Since the dementia is a prevalent disabling condition, it is essential to maintain their independence as long as possible to maintain a sound quality of life in individuals with dementia. Nonpharmacological intervention such as exercise has been introduced in one way which can help slow the progression of the disabling cascade and promote independence in people with dementia living in a long-term facility such as care hospitals.
Several studies have suggested beneficial effect of physical activity on physical functions and activities of daily living for people with dementia [1-6]. However, a review of interventions for people with dementia suggested that adherence to exercise interventions can often become more difficult as the severity of cognitive impairment increases [7,8] reported an adherence rate of 57% in a 16-week walking, while indicated a rate of 37% in a 12-month study on the effects of moderate exercise on daily life activities in institutionalized individuals with Alzheimer’s disease [9].
For exercise programs to be beneficial, having more help to deliver the exercise intervention longer periods and have the strategies to improve adherence [10]. However, there is a lack of data about long-term adherence effect of exercise training, detraining, and retraining is limited.
Therefore, this study aims to investigate the effect of the eighteen months of periodical exercise training and retraining after detraining on the physical function in older patients with dementia in South Korea.
Materials and Methods
Research design
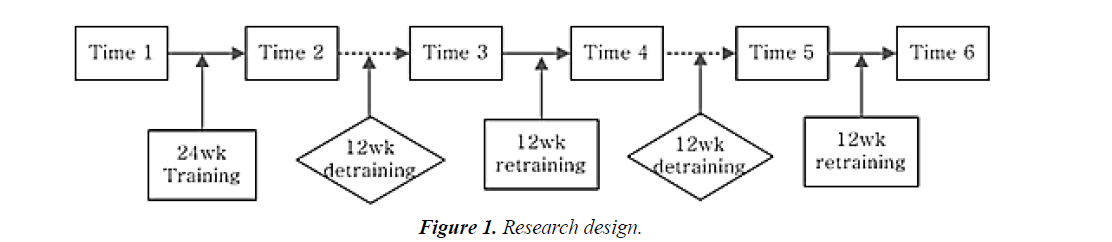
Training was undertaken in the initial 24-week training period and the 12-week retraining period. Further 12-week detraining and additional 12-week retraining was performed for 18 months (Figure 1).
Exercise intervention
Patients engaged in an individual exercise intervention by matching to trained college-student as exercise partners twice a week for the initial 24-week training period. Multi-component exercise intervention composed of aerobic exercise, strength, and flexibility exercise was conducted. Each class was 50- 60 minutes in length and began with 10 minutes of warm-up (range of motion) activities, followed by 30-40 minutes of multicomponent exercise, and finished with 10-minutes of relaxation activities.
Aerobic exercise was walking around a hospital for 15-60 minutes with RPE 10-15. Strength training consisted of the upper body using Thera-Band and dumbbells and the lower body using elastic bands. Resistance was set at 65% of their 1-repetition maximum (1RM) in the first week and 70% of 1RM in the second week and the after.
Upper body exercise includes chest press, seated row and biceps curl; lower body exercises consisted of knee extension, knee flexion, and leg press. Each exercise was performed for three sets of 8 to 15 repetitions. To progressively overload the muscle during the resistance exercises, participants were encouraged to increase the resistance of the elastic bands and dumbbells. When subjects could efficiently complete 15 repetitions of an exercise, they were encouraged to use a band of higher resistance, progressing from yellow to red to green to blue bands. A similar approach was used for the dumbbells, which ranged from one to three pounds.
Participants
Participants in this study were recruited through a collaborative agreement between the K University and G Geriatric hospital. Patients who met the following criteria were included: (a) over 65 year, (b) diagnosis of dementia, (c) a Mini-Mental State Examination (MMSE-K) score ≥ 10, (d) able to stand and walk for 30 m without shortness of breath, (e) ability to stand up from chair with armrests with assistance from ≤ 1 person, and (f) physicians approval. Before the start of the study, the patients or their family member were informed of the characteristics of the research project. Informed consent in written format was provided to the participants or their family. The study was approved by the Clinical Research Ethics Committee of K hospital (YI-2011-02).
Outcome measurers
1) Physiological measurement: Participants’ physiological variables-body weight, Body Mass Index, percent fat and fatfree mass were measured with InBody720 (InBody Co., Seoul).
2) Functional measurements:
Senior fitness test: The Senior Fitness Test [11] battery for older adults was used to assess functional fitness associated with maintaining physical independence in later life. The test items included: 1) sit to stand to assess lower-body strength; 2) arm curl test to measure upper-body strength; 3) 2 min step test- to assess aerobic endurance; 4) chair sit-and-reach test to assess lower-body flexibility; 5) back scratch test to assess upper body flexibility; and 6) 8-foot Time to up and go(TUG) test to assess agility and dynamic balance.
Short physical performance battery (SPPB): The SPPB is a valid and reliable performance test which includes three tests of physical function: balance, gait speed, and chair stand. Each of three tests were score 0 to 4 according to standardized criteria, with a score of 0 indicating an inability to complete the test and four the highest level of physical performance. Score from the three tests were summed into a final score ranging from 0 to 12, with higher scores reflecting better physical function.
4-meter walk: Gait speed was measured by asking patients from a standing still start position, to walk a distance of 4-meter at their usual pace. Patients are allowed to use a walking aid, if necessary. The outcome measure is the mean duration of 2 attempts. Walking speed is associated with adverse events and cognition in healthy older adults [12].
Cognitive function
The cognitive function for patients with dementia was measured by Korean version of the Mini-Mental State Exam (MMSE-K).
Statistics
All statistical analyses were conducted with the IBM Statistics 20.0(SPSS, Inc, IBM Company). Training, detraining and retraining data of the functional fitness test were treated with a nonparametric test Friedman's test first, and if the p-value was <0.05, Wilcoxon matched pairs signed rank test was used for examining paired differences. A p-value less than 0.05 was considered statistically significant. The data are expressed as mean ± SD.
Results
The main characteristic of the participants included in the study is shown on Table 1. The mean age of the participants was 74.46 ± 8.24 years with a body mass index of 27.28 ± 8.32 and 30.21 ± 3.17% of body fat. The fat-free mass was 39.34 ± 5.18, and MMSE-K was 17.8 ± 5.4, which is classified as a moderate dementia. Attendance rate was calculated by dividing the number of exercise sessions completed by participants by the full amount of session they were expected to perform through the study. The attendance rate was 1.49 ± 0.86 and average duration of aerobic exercise was 30.64 ± 20.24 minutes. The reasons for missing exercise session were unwillingness to participate in exercise session, behaviour disorders, and participate in special events.
Table 1. Main characteristics of participants.
| Characteristics | Value (Mean ± SD) or n (%) |
|---|---|
| Age (y) | 74.46 ± 8.24 |
| Gender (Men) | 7 (41.2) |
| Body weight (kg) | 27.28 ± 8.32 |
| BMI(kg/m2) | 23.01 ± 2.67 |
| SBP(mmHG) | 122.06 ± 13.09 |
| DBP(mmHG) | 70.94 ± 14.52 |
| Percent fat (%) | 30.21 ± 3.17 |
| FFM(kg) | 39.34 ± 5.18 |
| Waist-hip ratio | 0.98 ± 0.04 |
| BMR(kcal) | 1270.63 ± 116.88 |
| Waist (cm) | 91.31 ± 3.26 |
| MMSE-K (score) | 17.8 ± 5.4 |
All changes in physiological measure are displayed in Table 2. During the course of the study, body weight, Body Mass Index (BMI), percent body fat, fat free mass, Basal Metabolic Rate(BMR), waist were not significantly changed.
Table 2. Change of physiological measurement after periodical exercise training, detraining and retraining.
| Variable | Baseline | 24 weeks (training) | 36 weeks (detraining) | 48 weeks (retraining) | 60 weeks (detraining) | 72 weeks (retraining) | F |
|---|---|---|---|---|---|---|---|
| Weight, kg | 57.28 ± 8.32 | 60.45 ± 6.49 | 61.38 ± 6.13 | 62.42 ± 5.96 | 62.32 ± 6.50 | 62.27 ± 6.47 | 11.03 |
| BMI, kg/m2 | 23.01 ± 2.67 | 24.03 ± 2.60 | 24.47 ± 2.15 | 24.96 ± 2.31 | 24.64 ± 2.28 | 24.77 ± 2.26 | 10.19 |
| FFM, kg | 39.34 ± 5.18 | 40.29 ± 5.73 | 40.29 ± 5.19 | 40.91 ± 5.40 | 39.84 ± 5.57 | 41.14 ± 6.70 | 9.04 |
| Body fat, % | 30.21 ± 3.17 | 32.34 ± 2.87 | 33.21 ± 1.62 | 32.74 ± 2.43 | 32.99 ± 1.53 | 33.70 ± 1.76 | 8.306 |
| BMR, kcal | 1270.63 ± 116.88 | 1292.25 ± 128.75 | 1292.88 ± 116.32 | 1301.25 ± 121.25 | 1281.75 ± 125.72 | 1286.25 ± 118.86 | 8.61 |
| Waist, cm | 91.31 ± 3.26 | 90.54 ± 2.34 | 93.08 ± 2.55 | 90.38 ± 2.14 | 95.0 ± 1.44 | 92.75 ± 2.27 | 10.05 |
BMI: Body Mass Index; FFM: Fat Free Mass; BMR: Basal Metabolic Rate
As for detraining effects, trained-related improvements remained above baseline value for sit-to-stand, 8-foot TUG, and 4-meter walk (Table 3). The cessation of exercise for 12 weeks showed negative changes in sit to stand, 8-foot TUG, 4-meter walk and SPPB (p<0.05). Nevertheless, the resumption of multicomponent exercise training the above change recovered the negative effects and induced favourable functional adaptation after first 12 weeks of retraining.
Table 3. Change of physical function after periodical exercise training, detraining and retraining.
| Variable | Baseline1 | 24 weeks2 (training) | 36 weeks3 (detraining) | 48 weeks4 (retraining) | 60 weeks5 (detraining) | 72 weeks6 (retraining) | F | Comparison* |
|---|---|---|---|---|---|---|---|---|
| Sit to stand, times/30 sec | 9.12 ± 1.96 | 15.63 ± 4.72 | 11.50 ± 3.21 | 15.38 ± 5.66 | 12.13 ± 3.94 | 13.00 ± 2.45 | 16.52* | 1-2, 1-4, 1-5, 1-6, 3-6 |
| Arm curl, times/30 sec | 26.56 ± 2.04 | 27.67 ± 1.55 | 19.11 ± 1.87 | 24.89 ± 2.76 | 19.11 ± 1.02 | 23.11 ± 1.78 | 14.45* | 1-3, 1-5, 2-3, 2-5, 3-4, 3-6, 4-5, 5-6 |
| 8-foot TUG, sec | 13.94 ± 5.17 | 7.85 ± 1.85 | 11.42 ± 2.23 | 8.82 ± 1.63 | 9.65 ± 1.73 | 10.33 ± 3.89 | 15.85* | 1-2, 1-4, 1-5, 2-3 |
| Chair sit-and-reach, cm | -5.50 ± 12.04 | -7.19 ± 15.29 | -4.73 ± 6.47 | 4.13 ± 11.94 | -0.413 ± 10.06 | 1.31 ± 3.92 | 8.498* | |
| Back scratch test, cm | -28.94 ± 3.27 | -21.33 ± 2.92 | -19.44 ± 6.82 | -23.3 ± 3.12 | -22.16 ± 2.54 | -27.33 ± 3.45 | 4.968* | |
| 4 m walk, sec | 6.64 ± 2.54 | 4.05 ± 1.12 | 4.87 ± 1.40 | 3.85 ± 0.58 | 6.01 ± 1.39 | 5.89 ± 1.60 | 17.38* | 1-2, 1-4, 2-5, 2-6, 4-5, 4-6 |
| One leg stand, sec | 8.99 ± 6.94 | 10.55 ± 12.07 | 8.26 ± 9.18 | 5.47 ± 5.08 | 7.02 ± 10.12 | 4.79 ± 3.59 | 8.633* | |
| SPPB, score | 8.00 ± 1.79 | 11.17 ± 1.17 | 9.83 ± 1.17 | 10.50 ± 1.64 | 9.00 ± 1.10 | 7.50 ± 2.07 | 14.85* | 1-2, 1-4, 2-5, 2-6, 3-6, 4-6 |
| Grip strength, kg | 16.38 ± 6.89 | 19.69 ± 7.89 | 17.84 ± 6.69 | 18.11 ± 7.53 | 18.44 ± 5.62 | 17.33 ± 7.52 | 5.89 | |
| 2 mi steps, steps | 64.88 ± 33.37 | 92.63 ± 16.66 | 66.63 ± 33.63 | 91.88 ± 39.87 | 97.00 ± 20.82 | 93.00 ± 38.49 | 9.64 |
TUG: Time to Up and Go; SPPB: Short Physical Performance Battery
During the study, sit to stand were significantly changed (F=16.515, p=0.006). It significantly increased following 24- week training(p<0.05), decrease following first 12 weeks detraining, significantly increased again in 12-week retraining(at 48 weeks) after second 12 week detraining(p<0.05), and significantly increased following last 12-week retraining (p<0.05). 8-foot TUG was significantly changed during the study (F=15.845, p=0.007). It significantly improved following first 24-week training (p<0.05), and significantly negative change in first 12-week detraining (at 36-week). It significantly improved second 12-week retraining (p<0.05) and adverse change in second 12-week detraining. Although it was not statistically significant, the final 8-foot TUG was positively changed compared to baseline data.
4-meter walk speed significantly changed throughout training, detraining and retraining cycle. (F= 17.377, p=0.004). It significantly improved following 24-week training (p<0.05), and significantly increased again in 12-week retraining after first 12-week detraining (p<0.05). For SPPB, it increased from 8.00 to 11.17 at 24 weeks (p<0.05). Cessation of periodic exercise for three months, however, resulted in a complete loss of the positive exercise-induced adaptation and reduced SPPB to that of the pre-exercise level(p<0.05). Twelve-week retraining increased SPPB to 10.53, however, continuously decreased in the additional 12-week detraining and 12-week retraining. Across the six time points, there was no significant change in sit and reach, back scratch, one leg stand, grip strength, and 2-minute step test.
Discussion
The present study tested and confirms that the effect of periodical multicomponent exercise training and detraining improved or maintained physical function in older adults with dementia. These results are supported by previous studies that have shown significant improvement in aerobic and strength capacity in demented older adults [13,14]. The improvements observed in the present study have significance for functional fitness which has consistently been linked with better health status, the ability to perform an activity of daily living and consequently with better quality of life.
The most novel finding for this study as that it demonstrated that multi-component exercise program promoted a significant increase in lower body strength, upper body strength, agility and dynamic balance and gait speed after retraining period in elders with dementia. These results indicate that individuals with dementia while they stop exercise worsened in almost all physical variables assessed. It regained, however, after they participated in retraining. Previous studies with general older subjects presented one year of detraining results in total loss of muscular adaptation in all variables [15]. We acknowledge the lack of a control group as a serious limitation of the present study to generalize our results. Age-related loss of muscle mass and strength are magnified by the lack of physical activity opportunities in the institutional settings leading to frailty faster. To our knowledge, the study presented herein include one of the most prolonged exercise intervention including trainingdetraining- retraining frame targeting older dementia subjects in South Korea.
Further, it is more practical research that reflects real exercise environments in the long-term care institution in that of periodical cessation of exercise program which automatically causes detraining and retraining. It was reported that most exercise interventions were led by volunteers or trained nonexercise specialist such as college students because of the budget issue [16-20]. With this kind of exercise instructors, it is hard to perform exercise program continuously because of a summer and winter vacation. Further, unlike general older population, aerobic exercise such as walking can be problematic for elders with dementia because it depends on the stage of the disease as well as other conditions such as hot or cold weather. Therefore, we can conclude that this research has identified 'feasible' yet 'effective' physical activity program that utilize minimal investment in staff and equipment, and demonstrated positive outcome even with cessation period. Implementation of such program represents cost-effective meas of providing long-term dementia elders with meaningful gain in physical and social function.
Conclusion
The present study tested and confirms that the effect of periodical multicomponent exercise training and detraining improved or maintained physical function in older adults with dementia. Due to the frailty of the participants, improvement of physical function contributes to a better performance of Activity daily living and delay the deterioration inherent in dementia.
References
- Hernández SS, Sandreschi PF, da Silva FC, et al. What are the benefits of exercise for Alzheimer’s disease? A systematic review of the past 10 years. J Aging Phy Activity. 2015;23(4):659-68.
- Rao AK, Chou A, Bursley B, et al. Systematic review of the effects of exercise on activities of daily living in people with Alzheimer’s disease. American J Occupational Therapy. 2014;68(1):50-6.
- Forbes D, Forbes C, Blake C, et al. Exercise programs for people with dementia. The Cochrane Library. 2015.
- Bossers WJ, Woude LH, Boersma F, et al. Comparison of Effect of Two Exercise Programs on Activities of Daily Living in Individuals with Dementia: A 9‐Week Randomized, Controlled Trial. J American Geriatrics Society. 2016;64(6);1258-66.
- Barreto P, Demougeot L, Pillard F, et al. Exercise training for managing behavioral and psychological symptoms in people with dementia: a systematic review and meta-analysis. Ageing Res Rev. 2015;24: 274-85.
- Kim K, Lee E. Effects of Cognition Improvement Programs on Normal Elderly in Korea: A Systematic Review and Meta-Analysis. Korea J Gerontol. 2017;37:431-44.
- Tilly J, P Reed. Falls, wandering, and physical restraints: a review of interventions for individuals with dementia in assisted living and nursing homes. Alzheimer's Care Today. 2008;9(1):45-50.
- Tappen RM, KE Roach, Applegate EB, et al. Effect of a combined walking and conversation intervention on functional mobility of nursing home residents with Alzheimer disease. Alzheimer Disease Associated Disorders. 2000;14(4): 196.
- Rolland Y, Pillard F, Klapouszczak A, et al. Exercise Program for Nursing Home Residents with Alzheimer's Disease: A 1‐Year Randomized, Controlled Trial. J American Geriatrics Society. 2007;55(2):158-65.
- Leung P, Yates L, Orgeta V, et al. The experiences of people with dementia and their carers participating in individual cognitive stimulation therapy. Int J Geriatric Psychiatry. 2017;32(12):e34-e42.
- Rikli R, Jones J. Development and Validation of Criterion-Referenced Clinically Relevant Fitness Standards for Maintaining Physical Independence in Later Years. Gerontologist. 2012;53(2):255-67.
- Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol Series A: Biological Sci Med Sci. 2005;60(10): 1304-09.
- Taguchi N, Higaki Y, Inoue S, et al. Effects of a 12-month multicomponent exercise program on physical performance, daily physical activity, and quality of life in very elderly people with minor disabilities: an intervention study. J Epidemiol. 2010;20(1):21-29.
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Archives of Physical Med Rehabilitation. 2004;85(10): 1694-704.
- Correa CS, Cunha G, Marques N, et al. Effects of strength training, detraining and retraining in muscle strength, hypertrophy and functional tasks in older female adults. Clin Physiol Functional Imaging. 2016;36(4):306-310.
- Shakeel S, Newhouse I, Malik A, et al. Identifying feasible physical activity programs for long-term care homes in the Ontario context. Canadian Geriatrics J. 2015;18(2):73.
- Bossers W, Scherder E, Boersma F, et al. Feasibility of a combined aerobic and strength training program and its effects on cognitive and physical function in institutionalized dementia patients. A pilot study. PloS one. 2014;9(5):e97577.
- Brett L, Traynor V, Stapley P. Effects of physical exercise on health and well-being of individuals living with a dementia in nursing homes: A systematic review. J American Med Directors Association, 2016;17(2):104-116.
- National Institute of Dementia (2017). Dementia Observatory 2016.
- van der VW, Jennie H, Dawid G, et al. Adherence support strategies for exercise interventions in people with mild cognitive impairment and dementia: A systematic review. Preventive Medicine Report. 2017;7:38-45.