Research Article - Biomedical Research (2020) Volume 31, Issue 5
Effects of Ivermectin-azithromycin-cholecalciferol combined therapy on COVID-19 infected patients: A proof of concept study
Guadalupe Espitia-Hernandez, Levy Munguia, Dylan Diaz-Chiguer, Ramiro Lopez-Elizalde, Fiacro Jimenez-Ponce*
Direccion Normativa de Salud, ISSSTE, Mexico City, Mexico
Accepted date: August 15, 2020
Abstract
As the world faces COVID-19, the search for effective treatments against the disease and its complications has turned its gaze to drugs that are classically used in other infectious diseases. Some drugs are being examined for the recent evidence on its effects on viral replication and inflammation. The aim of the study was to assess the outcome of Ivermectin-Azithromycin-Cholecalciferol combination therapy on early stages (I-IIa) of COVID-19 patients. This proof of concept study was carried out on confirmed COVID-19 patients in Mexico City, from April 01 to April 20, 2020. Patients who met inclusion criteria were invited to take Ivermectin (6 mg once daily in day 0,1,7 and 8) plus Azithromycin (500 mg once daily for 4 days) plus Cholecalciferol (4000 UI twice daily for 30 days). Treatment outcome was evaluated on the 10th day onward from the first day of the drug intake. Recovery rate of the 28 patients that received the combination therapy was 100%, the mean symptomatic recovery duration was 3.6 days and negative PCR was confirmed on day 10. Imaging findings of patients with pneumonia were improved on day 10. Transient and mild degree of adverse effect like diarrhea and nausea was noted by 3 (10.7%) patients. This study found that the combination treatment might mitigate disease progression without significant adverse effects. Further studies are needed in order to extrapolate these findings to moderate and severe COVID-19.
Keywords
SARS-CoV-2, COVID-19, Ivermectin, Azithromycin, Cholecalciferol.
Introduction
Currently, the world is in a race against time to curb the human and economic losses for which the SARS-Cov-2 virus is responsible. This virus infection was initially classified as pneumonia of unknown origin to be later recognized as COVID-19 [1]. The symptoms of COVID-19 infection appear after an incubation period of approximately 5 days with a median of 14 days to develop complications and death [2]. The progression depends on age and comorbidities, where a higher speed of progression is observed in patients>70-years old [3]. The spectrum of symptoms includes fever, cough, headache, diarrhea, dyspnea, anosmia and dysgeusia. Imaging studies reveal findings of pneumonia with grand-glass opacities, acute respiratory distress syndrome and acute cardiac injury [4,5].
Scientists around the world are actively exploring drugs that could be potentially effective in combating COVID-19. Recent publications have drawn attention to the possible benefits of ivermectin, azithromycin and cholecalciferol. These drugs have been used for the treatment for infectious diseases since they have anti-inflammatory and antiviral properties [6,7]. The in vitro antiviral activity of Ivermectin has been related to inhibition of IMPα/1-mediated nuclear import of viral proteins [8]. The effect of azithromycin may be due to its properties as a weak base that could potentially block endocytosis and thereby limiting viral replication, by amplifying host ’ s interferon (IFN) pathway or by interfering with the SARS-CoV- 2 spike protein and host receptor ACE2 (angiotensin converting enzyme-2) interaction inhibiting viral entry into the cells [9]. On the other hand, in viral infections, cholecalciferol may decrease pro-inflammatory cytokines associated with the Th1 type response and increase anti-inflammatory cytokines associated with the Th2 type immune response [10]. Therefore, we decided to evaluate the efficacy of the Ivermectin- Azithromycin-Cholecalciferol combination and compare it to the standard therapy provided to patients in early stages of COVID-19.
Materials and Methods
Eligibility criteria
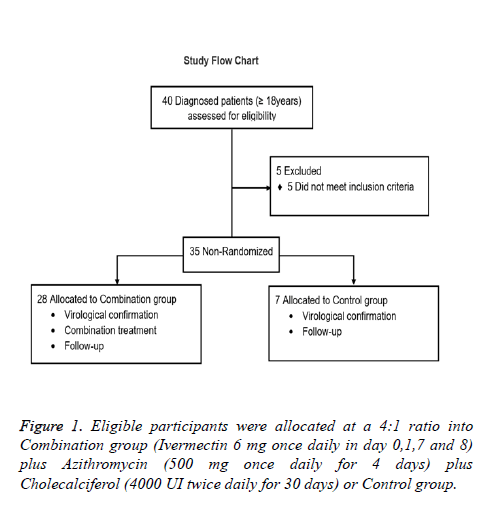
Eligible subjects were; 18 years old and older with a laboratory confirmed diagnosis of SARS-CoV2 using the CDC reverse transcription–polymerase chain reaction SARS-CoV-2 assay, with mild (cough, fever, malaise, myalgias, gastrointestinal symptoms, ageusia and anosmia) or moderate (dyspnea, tachypnea, hypoxemia, and abnormal lung findings) COVID-19 symptomatology. Patients with severe comorbid conditions like severe Bronchial asthma, COPD, severe ischemic heart disease, uncontrolled diabetes mellitus, advanced renal and hepatic disease, carcinoma, immuno-compromised or allergic to any of the drugs treatment were not included in this study. The flow chart (Figure 1) presents patients distribution.
Study design
This was a proof-of-concept study for the evaluation of clinical efficacy of Ivermectin, Azithromycin and Cholecalciferol combination in the treatment of new coronavirus (COVID-19). 35 adult patients who were tested positive for SARS-CoV-2 infection by RT PCR were included. The study was conducted in Mexico City, between April 1 and May 20, 2020. For the study purpose patients are presented as follows: Patients were voluntarily allocated into 2 groups at a 4:1 ratio; 1) Combination group (n=28): Ivermectin (6 mg once daily in day 0,1,7 and 8) plus Azithromycin (500 mg once daily for 4 days) plus Cholecalciferol (4000 UI twice daily for 30 days) plus standard treatment and 2) Control group (n=7): Standard treatment (self-isolation, proper nutrition, oral hydration and acetaminophen). Subjects enrolled in this study were treated as outpatients and received information about the correct use of medication and were kept informed about the course of their disease. They were instructed when to look for help in case of worsening of symptoms or pulmonary deterioration as recorded by daily pulse oximeter oxygen saturation (SpO2) measurements (thrice a day). All patients provided written informed consent. The study was approved by the Institutional Committees of the ISSSTE and was conducted according to the principles of the Declaration of Helsinki. Data were collected and analyzed by the investigators. This study is reported in accordance with the EQUATOR Reporting Guidelines.
Outcomes
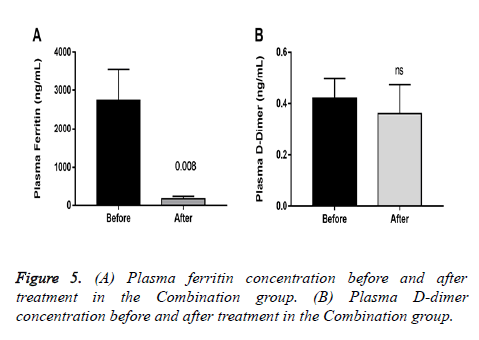
The primary end point was the efficacy of the combination therapy. A negative PCR was counted as treatment success. Secondary end points included the duration from the first-day drug intake to the alleviation of clinically significant symptoms of COVID-19, temperature and ventilatory responsiveness. Regular contacts were maintained to find out the adverse or side effects of the therapy. Patients with pneumonia in the combination group (13 of 16) were tested for laboratory changes in D-dimer and ferritin comparing before and after treatment to analyse changes related to hypercoagulability and inflammation.
Statistical analysis
Data was expressed as mean ± SD for continuous variables and percentage for categorical variables description. Descriptive statistics were used for demographic and clinical data. We used paired-t test with Welch correction to compare means for intragroup analysis. Statistical significance was considered when p 0.05. Analyses were performed with Graph Pad Prism, v7.0.
Results
Characteristics
Basal clinical characteristics of patients are presented in Table 1. An overall mean of 45 ± 10 years of age. Obesity (34%) was the most frequent comorbidity. Acetaminophen was prescribed for all patients for temperature control; however, the frequency of use was only of 28.6% and 42.8% in the combination and control groups, respectively.
| Variable | Number | Combination group (n=28) | Control group (n=7) | |
|---|---|---|---|---|
| Age, mean (SD) | 45.1 (10.1) | 44 (10.5) | 49.7 (7.3) | |
| Men (%) | 16 (45.7) | 12 (42.8) | 4 (57.1) | |
| Comorbidities | ||||
| Obesity, number (%) | 11 | 8 | 3 | |
| Diabetes mellitus, number (%) | 2 | 2 | 0 | |
| Hypertension, number (%) | 1 | 1 | 0 | |
| Asthma, number (%) | 3 | 3 | 0 | |
| COPD, number (%) | 1 | 0 | 1 | |
| Allergic rhinitis, number (%) | 1 | 0 | 1 | |
| Heart disease, number (%) | 1 | 1 | 0 | |
| Coarctation of the aorta, number (%) | 1 | 1 | 0 | |
| Von Willebrand disease, number (%) | 1 | 1 | 0 | |
| Rheumatoid arthritis, number (%) | 2 | 1 | 1 | |
| Vitiligo, number (%) | 2 | 2 | 0 | |
| Renal lithiasis, number (%) | 1 | 1 | 0 | |
| Body weight, mean (SD), kg | 73.4 (11.5) | 70.5 (11.2) | 75 (14.7) | |
| Body temperature, mean (SD),°C | 37.1 (1.3) | 36.9 (1.2) | 37.5 (1.6) | |
| Heart rate, mean (SD), bpm | 82 (19) | 82 (18) | 88 (28) | |
| Oxygen saturation, mean (SD), % | 93 (4) | 94 (3) | 92 (5) | |
| SpO2/FiO2 ratio, mean (SD) | 443.5 (19.1) | 447.44 (12.7) | 439.5 (24.3) | |
| Pneumonia, number (%) | 18 (51.4) | 16 (57.1) | 2 (28.6) | |
| Symptoms | ||||
| Odynophagia, number (%) | 27 (77.2) | 24 (85.7) | 3 (42.9) | |
| Cephalea, number (%) | 20 (57.2) | 16 (57.2) | 4 (57.2) | |
| Asthenia/Adynamia, number (%) | 19 (54.3) | 17 (60.7) | 2 (28.6) | |
| Cough, number (%)( | 17 (48.6) | 14 (50) | 3 (42.9) | |
| Fever, number (%) | 16 (45.8) | 13 (46.5) | 3 (42.9) | |
| Arthralgias, number (%) | 14 (40) | 13 (46.5) | 1 (14.3) | |
| Diarrhea, number (%) | 7 (20) | 6 (21.5) | 1 (14.3) | |
| Conjunctivitis, number (%) | 6 (17.2) | 5 (14.3) | 1 (14.3) | |
| Rhinorrhea, number (%) | 4 (11.5) | 3 (10.7) | 1 (14.3) | |
| Asymptomatic, number (%) | 3 (8.6) | 3 (14.3) | 0 (0) | |
| Expectoration, number (%) | 2 (5.8) | 2 (7.14) | 0 (0) | |
| Pulmonary X-ray | ||||
| Not consistent with pneumonia | 17 (48.6) | 12 (42.9) | 5 (71.4) | |
| Consistent with pneumonia | 18 (51.4) | 16 (57.1) | 2 (28.6) | |
Table 1: Baseline from demographic, clinical, image and laboratory.
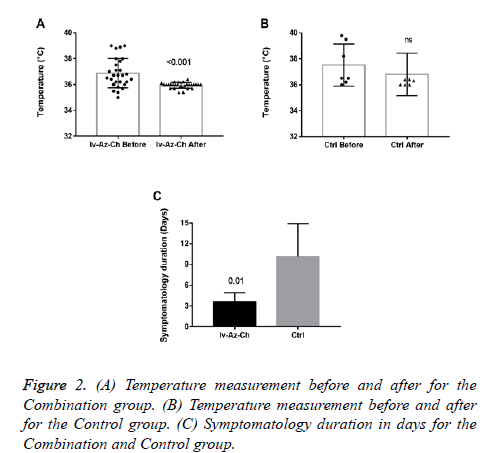
Results showed a significant reduction on body temperature (-0.94 ± 0.2°C, p<0.001) in the combination group with no significant difference in the control group (Figures 2A and 2B). Also, a significant reduction in the duration of symptoms was found in the combination group as compared with control group (3 ± 1.3 vs. 10 ± 4.8, respectively) (Figure 2C).
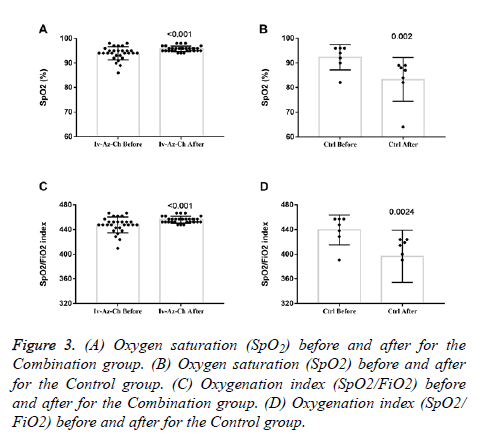
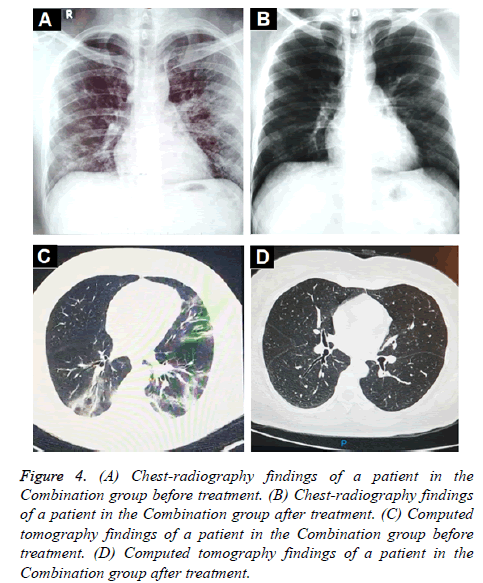
Oxygen saturation (SpO2) and oxygenation index (SpO2/FiO2) showed a small but significant increase in the combination group (Figures 3A and 3C) whereas a significant decrease was found in the control group (Figures 3B and 3D). In terms of radiologic findings, bilateral pneumonia was more prevalent in the Combination group when compared with control (16 of 28 patients [57.1%] vs. 2 of 7 patients [28.6%]). For the combination group, chest radiography (28 of 28) and CT-scan (6 of 6) significantly reduced the multiple mottling and ground-glass opacity after treatment (Figure 4).
Figure 3: (A) Oxygen saturation (SpO2) before and after for the Combination group. (B) Oxygen saturation (SpO2) before and after for the Control group. (C) Oxygenation index (SpO2/FiO2) before and after for the Combination group. (D) Oxygenation index (SpO2/ FiO2) before and after for the Control group.
Figure 4: (A) Chest-radiography findings of a patient in the Combination group before treatment. (B) Chest-radiography findings of a patient in the Combination group after treatment. (C) Computed tomography findings of a patient in the Combination group before treatment. (D) Computed tomography findings of a patient in the Combination group after treatment.
All patients in the combination group achieved a negative PCR on day 10, whereas the control group remained positive. After treatment, plasmatic ferritin was significantly reduced in patients in the combination group with pneumonia, whereas D-dimer showed no differences (Figure 5). In the Control group, 6 (85.7%) patients continued with progression of symptoms on day 7, and 1 patient required hospitalization.
Safety outcomes
Transient and mild adverse reactions like diarrhea and nausea have been reported in 3 (10.7%) patients in the combination group.
Discussion
In this proof of concept, we show that Ivermectin, Azithromycin and Cholecalciferol combination in an outpatient regimen is efficient in clearing viral nasopharyngeal carriage of SARS-CoV-2 in COVID-19 patients. A significant difference was observed in the combination group in the duration of symptoms and ventilatory responsiveness versus standard therapy. These results are of great importance since ambulatory treatment reduces the burden on the health system and the risk of contagion for others.
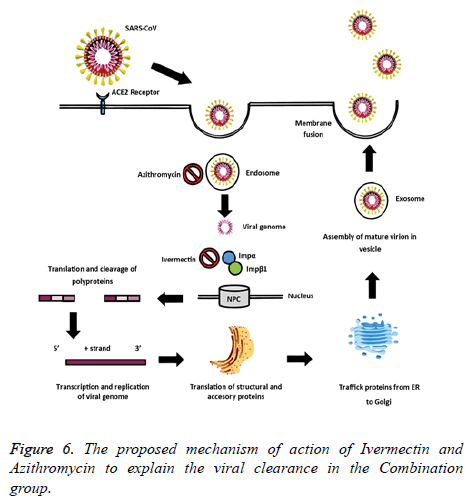
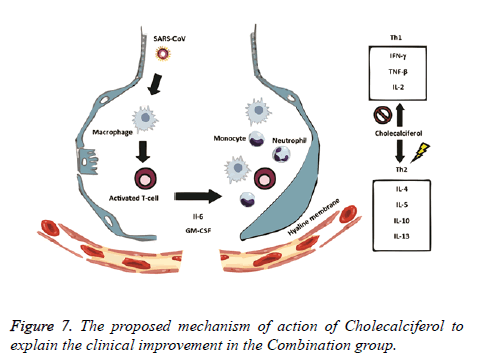
The rationale behind the combination and doses used were based on evidence from in vitro studies on the antiviral activity. Ivermectin significantly reduced SARS-CoV-2 RNA in cell cultures within 48 h [8]. Also, Ivermectin could block a viral non-structural protein (Nsp7) responsible for the replication and transcription of the viral genome [11,12]. On the other hand, Vitamin D could bind to the viral spike glycoprotein interfering with fusion of viral and host membranes [11], and it may also induce clinical improvements by attenuating lung injury and reducing epithelial cell apoptosis [13]. Azithromycin may block fusion of viral and endosome membranes by acidifying pH [14], and delay pulmonary fibrosis by decreasing viral replication in bronchial epithelium [15]. The benefits for mild to moderate COVID-19 patients were thought to be superior to the potential adverse effects of the combination. The proposed mechanisms of action of Ivermectin, Azithromycin and Cholecalciferol to explain the viral clearance and clinical improvement are illustrated in Figures 6 and 7.
Interestingly, elevated ferritin is associated with decreased lung function [16], our results showed a significant reduction in plasma concentration in a sample of the patients with pneumonia in the combination group was found (Figure 5).
Although highly preliminary and probably not sufficiently powered to be conclusive, this proof of concept results supported an effort to evaluate the effect of the proposed combination on the evolution and prognosis of COVID-19 more thoroughly. These findings should be further explored to know whether the combination is effective in severe cases.
Conclusion
This study has limitations, including its small sample size; due to its outpatient design, laboratories and CT-scans, to limit contact with reference COVID-19 hospitals, were not obtained from all patients. Radiologic and complete efficacy data will be presented later. In conclusion, in this study, a combined therapy with Ivermectin-Azithromycin-Cholecalciferol given for 7 days was effective to reduce symptomatology duration and clinical progression of COVID-19.
References
- Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, Iosifidis C, Agha R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg 2020.
- Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med 2020; 172: 577-582.
- Sun P, Lu X, Xu C, Sun W, Pan BJJomv. Understanding of COVID‐19 based on current evidence. 2020; 92: 548-551
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 2020; 395: 1054-1062.
- Xydakis MS, Dehgani-Mobaraki P, Holbrook EH, Geisthoff UW, Bauer C, Hautefort C, Herman P, Manley GT, Lyon DM, Hopkins C. Smell and taste dysfunction in patients with COVID-19. Lancet Infect Dis 2020.
- Choudhary R, Sharma AK. Potential use of hydroxychloroquine, ivermectin and azithromycin drugs in fighting COVID-19: trends, scope and relevance. Microbes Infect 2020; 35: 100684.
- Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging ClinExp Res 2020; 6: 1-4.
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 2020; 178: 104787.
- Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B. Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19. Clin Pharmacol Ther 2020.
- Hribar CA, Cobbold PH, Church FC. Potential Role of Vitamin D in the Elderly to Resist COVID-19 and to Slow Progression of Parkinson’s disease. Brain Sciences 2020; 10: 284.
- Dasgupta J, Sen U, Bakshi A, Dasgupta A, Manna K, Saha C, De RK, Mukhopadhyay S, Bhattacharyya NP. Nsp7 and Spike Glycoprotein of SARS-CoV-2 are envisaged as Potential Targets of Vitamin D and Ivermectin 2020.
- Kirchdoerfer RN, Ward AB. Structure of the SARS-CoV NSP12 polymerase bound to NSP7 and NSP8 co-factors2019. Nat commun 2019; 10: 1-9.
- Zheng S, Yang J, Hu X, Li M, Wang Q, Dancer RC, Parekh D, Gao-Smith F, Thickett DR, Jin S. Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-β induced epithelial to mesenchymal transition. Biochem Pharmacol 2020; 177: 113955.
- DerendorfHJIJoAA. Excessive lysosomal ion-trapping of hydroxychloroquine and azithromycin. 2020: 106007.
- Ulrich H, Pillat MM. CD147 as a Target for COVID-19 Treatment: Suggested Effects of Azithromycin and Stem Cell Engagement. Stem Cell Rev Rep 2020: 1-7.
- Lee J, kyeong Park H, Kwon MJ, Ham SY, Kim JM, Lim SY, Song JU. Decreased lung function is associated with elevated ferritin but not iron or transferrin saturation in 42,927 healthy Korean men: A cross-sectional study. PLOS ONE 2020; 15: e0231057