- Biomedical Research (2012) Volume 23, Issue 1
Effects of intraperitoneal glycerol and flax oil administration on colonic anastomosis healing
Erhan Aysan*, Serkan Sari, Hasan Bektas, Feyzullah Ersoz , Pınar Atukeren and Mahmut MuslumanogluBezmialem Vakif University, Vatan Caddesi, Fatih, Istanbul, Turkey
Accepted date: November 20 2011
Abstract
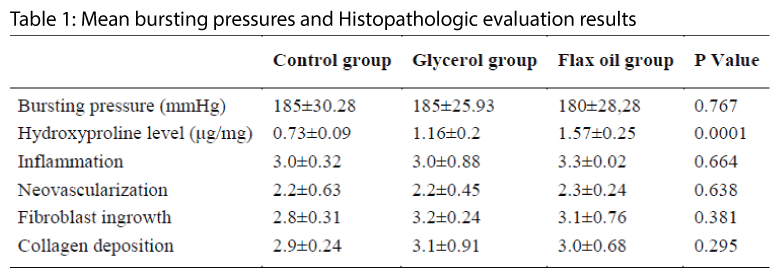
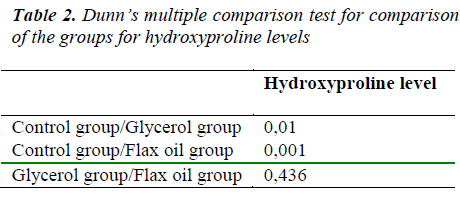
Effects of Intraperitoneal glycerol and flax oil on colonic anastomosis healing process was studied. Thirty female Wistar albino rats were divided into three groups. Each rat underwent segmental colonic resection with single-layer anastomosis. In group-1, the anastomotic line was covered with 9%NaCl, in group-2 with glycerol, and in group-3 with flax oil. The rats were sacrificed on postoperative day 10. Anastomosis burst pressures, tissue hydoxyproline levels and anastomosis histopathological characteristics were evaluated. We observed no statistical differences among all groups in mean bursting pressures (p= 0.767). The mean hydroxyprolin level of group 1 was statistically lower than group 2 and 3 (p=0.0001) but no difference between them 3 (p=0.436). Histopathologic evaluation results were not statistically different in all groups. The p values were 0.664 for inflammation, 0.638 for neovascularization, 0.381 for fibroblast ingrowth, and 0.295 for collagen deposition. Glycerol and flax oil are not harmful to the colonic anastomosis healing process. On the contrary, they increase mean tissue levels of hydroxyproline, which is directly related to anastostomotic healing. These liquids securely applicable for colonic anastomosis performed peritoneal cavities.
Keywords
Glycerol, colonic anastomosis, hydroxyproline, inflammation
Introduction
There are some complications of intestinal anastomoses but the three most common major complications are leakage, bleeding, and stenosis. Leakage is the most severe and common complication, and is associated with the highest mortality rate [1]. A multi-center study reported that the frequency of leakage from anastomoses following colonic resection was 0.5-30% [2]. More than half of all postoperative deaths are caused by sepsis associated with anastomotic complications [3,4].
Glycerol (synonym: glycerine, glycol alcohol) is a liquid alcohol. It dissolves in water and alcohols, but not in liquid hydrocarbons [5]. Being one of the most common molecules in living organisms, it is also a central component of lipids. Most fats consist of one molecule of glycerol combined with three molecules of fatty acids [6,7)].
Flax is a plant. Flax oil is derived from the seeds of the plant. The major fatty acid in flax oil is alpha-linolenic acid. Flax is rich in gamma-tocopherol (8). Because of its rich omega-3 fatty acids, flax oil has antiproliferative, antiinflammatory, antiedema and antioxidant effects [9,10]).
The team of authors has been working on prevention of postoperative peritoneal adhesions (PPA) for years. During this long period of time, after many materials were investigated [11-15] we found that glycerol and flax oil were the most effective materials for prevention of PPA [14,15].
Because of the fact that gastrointestinal anastomoses complications are very important for surgical procedures, we need to examine the effects of these liquids on anastomosis healing. For this purpose, we performed a single-layer standard anastomosis on the proximal colon after segmental resection and compared the anastomotic healing with macroscopic and microscopic parameters.
Method
This study was performed at the Experimental Animal Production and Research Laboratory of Cerrahpasa Medical School, Istanbul University and was approved by the appropriate Animals Ethics Committee. All protocols were in accordance with the regulations governing the care and use of laboratory animals of the declaration of Helsinki.
Rats were divided into three equal groups, with a sample size power of 0.9, in a 95% confidence interval, which resulted in a distribution of 10 rats in each group. Thirty Wistar out-bred female albino rats (mean weight, 265 ± 12g; mean age, 6.5 months) were used. Approximately 10-12 hours before each operation, feeding boxes were replaced to assure gastric emptying during the period of surgery.
All rats were anesthesized with 40 mg/kg body weight, intramuscular Ketamine (Ketalar®, Parke Davis and Co. Inc.).
Method used in group 1 (control group)
After antisepsis was provided by povidone iodine (Betadine®, Kurtsan Co.), a 3cm long vertical median incision was made. A 2cm long proximal colonic segment was resected and a single-layer standard end-to-end anastomosis with 4/0 polypropylene suture (Prolen®, Bicakcilar Co. Turkey) was performed. After anastomosis, a 1 ml sterile 9%NaCl (Serum Fizyolojik®, Eczacibasi Co. Turkey) was applied to this area. Then the incision was closed with 3/0 polypropylene suture using a continuous suture technique. Rats were sacrificed 10 days postoperatively by intraperitoneal injection of 100 mg/kg sodium pentothal. To expose the entire peritoneal cavity, an inverted “U” incision was made on the anterior abdominal wall. The anastomotic contour was resected and burst pressure of the anastomosis were measured with a transducer (Alp-K2 SphygmomanometerÒ, Norticon Co.). Following the burst test, each anastomotic contour was cut out and divided into two segments. One segment was placed in formol for histopathological investigation. The other segment of the anastomotic contour was covered with aluminium folio and kept at -70°C in a freezer for tissue hydroxyproline determination with quantitatively determined spectrophotometrically at 560nm using the hydroxyproline kit (Hypronosticon, Organon, Holland) and the results were interpreted as μg/mg wet tissue.
Method used in group 2 (glycerol group)
All procedures were identical to those in group 1, except that, following the anastomoses performed, 1 ml sterile glycerol (Gliserin®, Adora Kimya Co. Turkey) applied to this area. Postoperative care, sacrifice, and subsequent procedures were as in group 1.
Method used in group 3 (flax oil group)
All procedures were identical to those in group 1, except that, following the anastomisis performed, 1 ml sterile flax oil (Keten yagi®, Arifoglu Co.Turkey) applied to this area. Postoperative care, sacrifice, and subsequent procedures were as in group 1.
In this research, the primary evaluation criteria were anastomotic burst pressure and tissue hydoxyproline levels, and the secondary criteria was histopathological evaluation results.
Statistical Evaluation
Statistical analyses were performed using NCSS 2007 software. Results were evaluated with a confidence interval of 95% and p<0.05. In addition to descriptive statistical methods (mean, standard deviation, median), the Kruskal Wallis test for intergroup comparisons, the Dunn’s multiple comparison test for comparison of subgroups, and the Chi-Square Test for comparison of nonparametric variables were used for evaluation of data.
Results
We observed no statistically differences among all groups in mean bursting pressures (p= 0.767, table-1). Mean hydroxyprolin level of group 1 (control group) was statistically low than group 2 and 3 (p=0.0001) but there was no difference between groups 2 and 3 (p=0.436, table-2). Histopathologic evaluation results were not statistically different in all groups (Table 1).
Discussion
Since 1999, we the team of authors have been focused on the use of viscous liquids to prevent PPAs. For this purpose we tested many viscous liquids [11-16]. Our first international manuscript was published in 2002 [16]. Because PPAs result from peritoneal trauma [17,18], there are two fundamental methods for preventing PPArelated complications: the prevention of adhesion formation, and the treatment of adhesions after they have formed. The second method is particularly complex because it is related to the wound healing process. These processes involves multiple cell populations, the extracellular matrix and the action of soluble mediators such as growth factors, and cytokines, with some steps and molecular actors still unclear [19]. We therefore focused on preventing PPAs by covering the peritoneal surface with viscous liquid(s) that do not have negative effects on vital tissues, especially peritoneal mesothelial cells. Covering peritoneal surfaces with viscous liquids may prevent the peritoneum from experiencing mechanical trauma, both by preventing direct contact with the trauma-inducing material and by spreading any focused pressure via fluid surface tension.
In this long time period of research we found that soybean oil [11], octyl methoxycinnamate [12], aloe vera jelly [13], glycerol [14] and flax oil [15] were effective in preventing PPAs. Multivariate analysis showed that application of these liquids into the peritonel cavity significantly decreased PPAs. Glycerol and flax oil were being the most effective liquids.
In this research, we aimed to evaluate the effects of glycerol and flax oils on the colonic anatomosis healing process because the abdominal surgeon must be sure of anastomotic healing security when glycerol or flax oil were used for prevention of PPAs intraperitoneally. It is clear that anastomotic leakage is the most important morbid and mortal complication in abdominal surgery [20-22]. At least one-third of deaths following colorectal surgery are attributed to anastomotic leakage. Anastomotic leakage is also the most important cause of increased hospital stay duration (21-23). Leakage rates vary greatly and are associated with 0 to 30% of all cases, but clinically detected leakage rates are between 2.1 and 14.9% [20,21].
For evaluation of anastomotic healing process, both mechanical and biochemical parameters are important. Mechanical parameters, either bursting pressure or breaking strength indicates the resistance of the anastomotic contour to intra-luminal pressure. The anastomosis burst pressure is directly related to the amount of mature connective tissue formed by collagenous tissue deposition [24]. The biochemical evaluation of anastomotic healing process has been limited to behavior of collagen. Collagens which guarantee tissue continuity in the tissue repair period contain high proportions of glycine, proline and hydroxyproline. Tissue hydroxyproline levels are important parameters in the tissue repair process [24,25].
For these purpose, in this research we evaluated the anastomosis healing process primarily using anastomotosis bursting pressure and tissue hydoxyproline levels. We found that no statistically differences among all groups in mean bursting pressures but mean hydroxyprolin level of groups glycerol and flax oil were higher the control group. Tissue levels of inflammation cells, fibroblast ingrowth, collagen deposition and neovascularization are other important parameters to the anastomotic healing process [25].
In conclusion, glycerol and flax oil as the new and successfully PPA prevention materials are not harmful to the colonic anastomosis healing process; on the contrary they increase mean tissue levels of hydroxyproline, which is directly related to anastostomotic healing. Our results conlude in clinical practice that, glycerol and flax oil are securely applicable for prevention of PPA also in colonic anastomosis performed peritoneal cavities. After local ethic committee approved and informed consent are required we author team are planning to use these liquids in second laparotomy indicated patients especially low anterior colonic resections with protective loop ileostomy performed.
References
- Jex RK, Van Heerden JA, Wolff BG, Ready RL, Ilstrup DM. Gastrointestinal anastomoses. Ann Surg 1987; 206; 138-141.
- Fielding LP, Stewart-Brown S, Bleosovski L, Kearney G. Anastomotic integriity after operations for large bowel cancer: a multicenter study. Br Med J 1980; 28: 411-414.
- Beard JD, Nicholson ML, Sayers RD, Lloyd D, Everson NW. Intraoperative air testing of colorectal anastomoses: a prospective, randomized trial. Br J Surg 1990; 77:1095-1097.
- Hansen O, Schwenk W, Hucke HP, Stock W. Colorectal stapled anastomoses. Experiences and results. Dis Colon Rectum. 1996; 39: 30-66.
- Ott L, Bicker M, Vogel H. Catalytic dehydration of glycerol in sub- and supercritical water: a new chemical process for acrolein production. Green Chem 2006; 8: 214-220.
- Watanabe M. Acrolein synthesis from glycerol in hot- compressed water. Bioresour Technol 2007; 98: 1285-1290.
- Yazdani SS, Gonzalez R. Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr Opin Biotechnol 2007; 18: 213-219.
- Bozan B, Temelli F. Chemical composition and oxidative stability of flax, safflower and poppy seed and seed oils. Bioresour Technol. 2008; 99: 6354-6359.
- Dupasquier CM, Dibrov E, Kneesh AL, Cheung PK, Lee KG, Alexander HK, Yeganeh BK, Moghadasian MH, Pierce GN. Dietary flaxseed inhibits atherosclerosis in the LDL receptor-deficient mouse in part through antiproliferative and anti- inflammatory actions. Am J Physiol Heart Circ Physiol. 2007; 293: 2394-2402.
- Singh S, Nair V, Jain S, Gupta YK. Evaluation of anti-inflammatory activity of plant lipids containing alpha-linolenic acid. Indian J Exp Biol 2008; 46: 453-456.
- Ayşan E, Bektaş H, Kaygusuz A. A new approach in decreasing postoperative peritoneal adhesions: preventing peritoneal trauma with soybean oil. J Inv Surg. 2009; 22: 275-280.
- Ayşan E, Bektaş H, Kaygusuz A. Efficacy of Octyl methoxycinnemate in preventing postoperative peritoneal adhesions: an experimental model. J Obst Gyneacol Res. 2009; 35: 1102-1108.
- Ayşan E, Bektaş H, Ersoz F. A new approach to postoperative peritoneal adhesions: Prevention of pritoneal trauma by aloe vera gel. Eur J Obstet Gynecol Reprod Biol. 2010; 149: 195-198.
- Ayşan E, Bektaş H, Kaygusuz A. Efficacy of glycerol in preventing postoperative peritoneal adhesions. J Obst Gyneacol Res 2010; 36 : 639-645.
- Ayşan E, Bektaş H, Kaygusuz A, Huq G.E. Eficacy of flax oil in peventing peritoneal adhesions. Eur Surg 2009; 41: 66-71
- Aysan E, Ayar E, Aren A, Cifter C. The role of intra- peritoneal honey administration in preventing post- operative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol. 2002; 104: 152-155.
- Senthilkumar MP, Dreyer JS. Peritoneal adhesions: pathogenesis, assessment and effects. Trop Gastroenterol 2006; 27: 11-18.
- Menzies D, Ellis H. Intestinal obstruction from adhesions-how big is the problem? Ann R Coll Surg Engl 1990; 72: 60-63.
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009; 37: 1528-1542.
- Schwab R, Weßendorf S, Gutcke A, Becker HP. Early bursting strength of human colon anastomoses - an in vitro study comparing current anastomotic techniques. Langenbeck’s Arch Surg. 2002; 386: 507-11.
- Månsson P, Zhang XW, Jeppsson B, Thorlacius H. Anastomotic healing in the rat colon: comparison between a radiological method, breaking strength and bursting pressure. Int J Colorectal Dis. 2002; 17: 420-425.
- Egger B, Inglin R, Zeeh J, Dirsch O, Huang Y, Büchler MW. Insulin-like growth factor I and truncated keratinocyte growth factor accelarate healing of left sided colonic anastomoses. Br J Surg. 2001; 88: 90-98.
- Shashidharan M, Lin KM, Ternent CA, Smyrk TC, Thorson AG, Blatchford GJ, et al. Influence of arginine dietary supplementation on healing colonic anastomosis in the rat. Dis Colon Rectum. 1999; 42: 1613-1617.
- Hendriks T, Mastboom WJ. Healing of experimental intestinal anastomoses. Parameters for repair. Dis Colon Rectum. 1990; 33: 891-901.
- Brown GL, Curtsinger LJ, White M, Mitchell RO, Pietsch J, Nordquist R, von Fraunhofer A, Schultz GS. Acceleration of tensile strength of incisions treated with EGF and TGF-beta. Ann Surg 1988; 208: 788-794