Research Article - Biomedical Research (2017) Volume 28, Issue 4
Effects of insulin therapy on glucagon in patients with newly diagnosed type 2 diabetes
Yuanyuan He1#, Bo Wu2#, Mei Meng1, Weiqing Ma1*, Defa Zhu2*1Department of Endocrinology, the Third Affiliated Hospital of Anhui Medical University, Hefei, China
2Department of Endocrinology, Institute of Anhui Geriatric, the First Affiliated Hospital of Anhui Medical University, Hefei, China
#These authors contributed equally for this work
- *Corresponding Author:
- Weiqing Ma
Department of Endocrinology
The Third Affiliated Hospital of Anhui Medical University, China
- *Corresponding Author:
- Defa Zhu
Department of Endocrinology, Institute of Anhui Geriatric
The First Affiliated Hospital of Anhui Medical University, China
Accepted date: September 29, 2016
Abstract
The aim of this study was to investigate the effects of insulin therapy on glucagon in patients with newly diagnosed Type 2 Diabetes (nd-T2DM patients). We recruited 93 nd-T2DM patients, including 45 nonobese patients and 48 obese patients. A 100 g bread meal test was performed before and after insulin therapy, and glucagon levels were measured before and after the experiment. Compared with the control group, the serum glucagon levels before treatment in both the obese and non-obese nd-T2DM patients were significantly higher (P=0.001). After treatment, the serum glucagon levels in the nd-T2DM patients had significantly decreased (P=0.001), but they were still higher than those in the control group (P=0.001). Additionally, the area under the curve of serum glucagon and postprandial glucagon levels in the non-obese nd-T2DM patients decreased significantly (P=0.001 and P<0.01, respectively). The serum glucagon level in the obese nd-T2DM patients decreased non-significantly (P>0.05). Insulin therapy improved serum glucagon levels in nd-T2DM patients. The serum glucagon level in the non-obese nd- T2DM patients improved significantly, but that in the obese nd-T2DM patients did not. The CP levels in the non-obese T2DM group at 30 min (P=0.003), 60 min (P=0.001), 120 min (P=0.001), and 180 min (P=0.001) after treatment had significantly increased compared to that before treatment. The CP levels in the obese T2DM group at 120 min (P=0.001) and 180 min (P=0.001) after treatment had significantly increased compared to that before treatment. This improvement might be related with a potential association between T2DM and obesity.
Keywords
Type 2 diabetes, Obesity, Glucagon, C peptide.
Introduction
Type 2 Diabetes Mellitus (T2DM) is a common metabolic disease characterized by a decline in insulin sensitivity and relative insulin insufficiency-induced hyperglycaemia [1]. Pancreatic β-cells secrete insulin, which is dependent on blood glucose levels. Insulin secretion is also influenced by paracrine interactions with surrounding cells, mainly α-cells and γ-cells. In normal physiological processes, stable fasting (basal) insulin and glucagon levels are maintained within a certain range, and insulin and glucagon work together to maintain stable blood glucose levels after an oral bread meal test. Patients with T2DM have defects in the secretion of both insulin and glucagon; they have low or high basal insulin levels, but their basal glucagon levels are increased or remain unchanged [2,3]. After the bread meal test, insulin is insufficiently secreted, and peak secretion is delayed; however, glucagon levels inappropriately decrease or increase [4-7]. Patients with T2DM and high basal insulin levels are often obese [8], but those with low basal insulin levels are usually non-obese. In addition to the abnormal secretion of insulin, the abnormal secretion of glucagon plays an important role in the occurrence and development of T2DM [9-15]. Abnormal glucagon secretion has different effects in non-obese and obese patients with T2DM. In non-obese patients with T2DM, basal glucagon levels are high or relatively normal [16,17], but postprandial glucagon levels are high [17]. Glucagon levels are believed to increase because fasting hyperglycaemia impairs glucagon secretion suppression, which is mediated by blood glucose levels [18]. Additionally, some studies reported that high blood glucose directly stimulates the secretion of glucagon [19]. Postprandial glucagon secretion is mainly regulated by insulin [20]. The main evidence for this is that, after consuming mixed meals, glucagon secretion is inhibited in individuals with normal insulin secretion, while the glucagon level increases in individuals whose postprandial insulin level does not increase, such as patients with type 1 diabetes mellitus [21]. In obese patients with T2DM, basal glucagon levels are higher [17], and the increase in bread meal-stimulated glucagon levels are more significant [17] than in non-obese patients with T2DM. Furthermore, the basal and postprandial glucagon levels are higher than in non-obese patients with T2DM [16,17,22,23]. Other studies suggested that the combination of T2DM and obesity is not related with the basal and postprandial glucagon levels observed in non-obese patients with T2DM [10]. During the treatment of patients with newly diagnosed T2DM (nd- T2DM), a variety of drugs can improve insulin secretion and basal and postprandial insulin levels [24,25]. Direct insulin therapy for patients with T2DM can not only directly improve insulin deficiency but also decrease glucagon levels; however, it cannot restore glucagon levels to normal levels [26-28]. It is still unclear whether insulin affects glucagon levels in nonobese and obese nd-T2DM patients. Therefore, this study investigated the effects of insulin therapy on glucagon levels in non-obese and obese nd-T2DM patients.
Materials and Methods
General data
A total of 93 nd-T2DM patients hospitalized in the Department of Endocrinology in the Third Affiliated Hospital of Anhui Medical University between February 2012 and November 2015 were selected; they had not received any medications. T2DM was confirmed for all patients according to the diagnostic and typing criteria for diabetes proposed by the World Health Organization (WHO) in 1999. Body Mass Index (BMI) was defined as weight (kg)/height (m2). According to the standards of obesity defined by the WHO guidelines for the Asian Pacific population (WHO/IASO/IOTF, 2000), these patients were divided into two groups: (1) non-obese T2DM group (BMI<25 kg/m2), which included 45 patients aged 37-75 years, and (2) obese T2DM group (BMI ≥ 25 kg/m2), which included 48 patients aged 21-77 years. This study was conducted in accordance with the Declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Anhui Medical University. Written informed consent was obtained from all participants. None of the patients had stress; cancer; autoimmune diseases; diabetic ketoacidosis; a hyperosmolar hyperglycaemic state; severe heart, liver, or lung diseases; or positive serum insulin antibodies and/or glutamic acid decarboxylase antibodies. Twelve age- and sex-matched healthy subjects were also included. General information about the study subjects is shown in Table 1.
| Index | Control | T2DM | P | Obese T2DM | P | Non-obese T2DM | P |
|---|---|---|---|---|---|---|---|
| Age (years) | 54.85 ± 11.62 | 54.95 ± 11.53 | 0.936a | 54.45 ± 12.24 | 0.718a | 55.50 ± 10.78 | 0.585a 0.527b |
| Gender(M/F) | 12 (7/5) | 93 (59/34) | - | 48 (32/16) | - | 45 (27/18) | - |
| BMI (kg/m2) | 22.25 ± 1.65 | 24.45 ± 3.08a | 0.001a | 26.82 ± 1.78a | 0.001a | 21.80 ± 1.77b | 0.001a 0.001b |
| TG (mmol/l) | 1.23 ± 0.56 | 2.47 ± 2.11a | 0.001a | 2.57 ± 1.80a | 0.001a | 2.28 ± 2.37a | 0.001a 0.381b |
| TC (mmol/l) | 4.6 ± 1.03 | 5.06 ± 1.30a | 0.001a | 5.21 ± 1.44a | 0.001a | 4.93 ± 1.13a | 0.001a 0.355b |
| HbAlC(%) | 4.95 ± 0.67 | 10.07 ± 2.57a | 0.001a | 10.30 ± 2.57a | 0.001a | 10.44 ± 2.55a | 0.001a 0.432b |
Table 1: Comparison of general conditions and biochemical indexes (͞x ± s).
Treatment of patients
Height and body weight were measured in the morning for all subjects. The serum Total Cholesterol (TC), Triglyceride (TG), and Haemoglobin A1 (HbAlC) levels were determined after sampling venous blood. A 100 g bread meal test was then performed, after which venous blood was sampled to measure blood glucose, C-Peptide (CP), and glucagon levels at different times (after fasting (T0) and 30 min (T1), 60 min (T2), 120 min (T3), and 180 min (T4) after the bread meal test). After admission, Novo Rapid 30 (insulin as part 30 injection) or glargine plus lispro insulin therapy was administered, and the dose was adjusted according to the blood glucose monitoring results, which were obtained using fingertip capillary blood samples. After glycaemic control was achieved (average treatment time, 13.5 ± 3.6 days), the 100 g bread meal test was performed after an overnight fast, and the indexes were measured again. This study applied individual glucose reducing programs; therefore, if some patients presented with symptoms of hypoglycaemia after achieving good blood glucose control, the blood glucose control range could be widened appropriately (≤ 8 mmol/l). Blood glucose was measured using the glucose oxidase method using venous blood samples and monitored using the Sure Step blood glucose meter (Johnson and Johnson, USA) for fingertip trace glucose. CP levels were detected using chemiluminescent immunoassay kits (Weifang 3V Bioengineering Group Co., Ltd., China). To measure glucagon, 2 ml venous blood was placed into a special anti-coagulated tube and stored at -18˚C after the plasma separated. Then, plasma glucagon levels were measured using radioimmunoassay kits (Atom Hi-tech Co. Ltd., China).
Statistical analysis
All data are expressed as mean ± Standard Deviation (SD). The Area Under the Curve (AUC) was calculated using the trapezoidal method, the independent samples t-test was used for intergroup comparisons, and the paired t-test was used for intragroup comparisons. P>0.05 was considered statistically significant. We used SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) for statistical analysis.
Results
Comparison of general conditions and related indexes before treatment
Except for age (P=0.936), the values for the remaining indexes in the T2DM group were all significantly higher than those in the control group (P=0.001). When comparing the non-obese T2DM group and control group, there were significant differences in HbAlC levels (P=0.001) and BMI (P=0.001), but no significant difference in age (P=0.585). The differences between the obese T2DM group and control group were also significant for HbAlC levels (P=0.001) and BMI (P=0.001), but not for age (P=0.718). When comparing the obese and nonobese T2DM groups, there were no significant differences in age (P=0.527) or HbAlC levels (P=0.432), but a significant difference in BMI (P=0.001) was observed. TC and TG levels in the obese and non-obese T2DM groups were significantly higher than in the control group (P=0.001, Table 1).
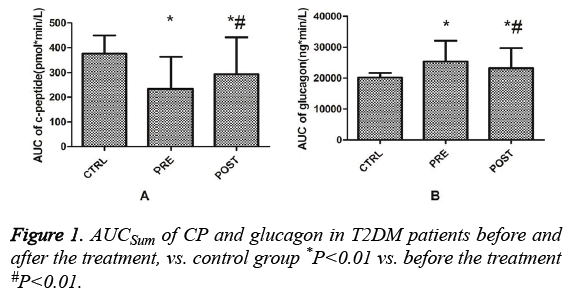
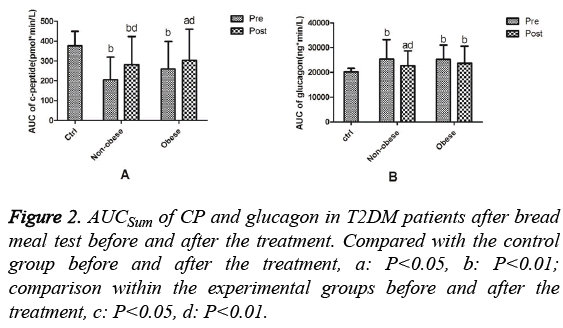
Comparison of the area under the curve (AUC) of Cpeptide and glucagon before and after treatment
Before treatment, the AUCSum (Sum of the Area Under the Curve) of CP in T2DM patients was significantly lower than that in the control group (P=0.001). After treatment, the AUCSum of CP in T2DM patients was lower than that in the control group (P=0.004), but significantly higher than that before treatment (P=0.001, Figure 1A). Before treatment, the AUCSum of glucagon in T2DM patients was higher than that in the control group (P=0.001). After treatment, the AUCSum of glucagon in T2DM patients was significantly higher than that in the control group (P=0.001) and significantly lower than that before treatment (P=0.001, Figure 1B). The AUCSum of CP before treatment in the non-obese T2DM group was significantly lower than that in the control group (P=0.001). After treatment, the AUCSum of CP was lower than that in the control group (P=0.003), but significantly higher than that before treatment (P=0.001). Pre-treatment, the AUCSum of CP in the obese T2DM group was significantly lower than that in the control group (P=0.007). Post-treatment, the AUCSum of CP was still lower than that in the control group (P=0.025), but significantly higher than that pre-treatment (P=0.001, Figure 2A). Before treatment, the AUCSum of glucagon in the nonobese (P=0.001) and obese (P=0.001) T2DM groups were significantly higher than that in the control group. After treatment, the AUCSum of glucagon in the non-obese T2DM group remained significantly higher than that in the control group (P=0.016), but decreased significantly compared to that before treatment (P=0.004). After treatment, the AUCSum of glucagon in the obese T2DM group remained significantly higher than that in the control group (P=0.002) and was not significantly different compared to that before treatment (P=0.05, Figure 2B).
Comparison of blood glucose, C-peptide, and glucagon levels at different times before and after treatment
Before treatment, the blood glucose levels in the non-obese and obese T2DM groups were significantly different from that in the control group at different times (P=0.001), but the differences between the non-obese and obese T2DM groups were not significant (P (T0)=0.713, P (T1)=0.968, P (T2)=0.580, P (T3)=0.197, P (T4)=0.263; Table 2). Before treatment, the CP levels in the non-obese T2DM group showed significant differences when compared with the control group at T1 (P=0.001), T2 (P=0.001), and T3 (P=0.001). The CP levels in the obese T2DM group were also significantly different from those in the control group at T1 (P=0.001), T2 (P=0.001), and T3 (P=0.040, Table 3). The glucagon levels in the non-obese and obese T2DM groups before treatment showed significant differences when compared with the control group at T1 (P=0.001, 0.001, respectively), T2 (P=0.001, 0.001, respectively), T3 (P=0.001, 0.001, respectively), and T4 (P=0.004, 0.002, respectively), but not at T0 (P=0.613, 0.087, respectively); however, the differences between the non-obese and obese T2DM groups were not significant (P (T0)=0.382 P (T1)=0.861, P (T2)=0.642, P (T3)=0.575, and P (T4)=0.680; Table 4).
| Control | Non-obese T2DM | Obese T2DM | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| Blood glucose | 0 | 4.71 ± 0.30 | 9.91 ± 2.58b | 7.31 ± 1.03bd | 9.72 ± 2.50b | 7.10 ± 1.18bd |
| Pb=0.001 | Pb=0.001, Pd=0.001 | Pb=0.001 | Pb=0.001, Pd=0.001 | |||
| 30 | 5.85 ± 0.28 | 13.27 ± 3.00b | 10.13 ± 1.83bd | 13.25 ± 3.04b | 9.80 ± 2.08bd | |
| Pb=0.001 | Pb=0.001, Pd=0.001 | Pb=0.001 | Pb=0.001, Pd=0.001 | |||
| 60 | 7.07 ± 0.22 | 17.45 ± 3.77b | 13.59 ± 2.48bd | 17.03 ± 3.59b | 12.89 ± 2.58bd | |
| Pb=0.001 | Pb=0.001, Pd=0.001 | Pb=0.001 | Pb=0.001, Pd=0.001 | |||
| 120 | 6.00 ± 0.31 | 19.85 ± 4.55b | 15.44 ± 3.19bd | 18.64 ± 4.44b | 14.14 ± 3.21bd | |
| Pb=0.001 | Pb=0.001, Pd=0.001 | Pb=0.001 | Pb=0.001, Pd=0.001 | |||
| 180 | 4.87 ± 0.32 | 18.03 ± 5.22b | 14.34 ± 3.76bd | 16.84 ± 4.99b | 12.01 ± 3.66bd | |
| Pb=0.001 | Pb=0.001, Pd=0.001 | Pb=0.001 | Pb=0.001, Pd=0.001 | |||
Table 2: Blood glucose at different time points before and after the treatment.
| Control | Non-obese T2DM | Obese T2DM | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| CP | 0 | 0.61 ± 0.12 | 0.52 ± 0.27 | 0.66 ± 0.30 | 0.68 ± 0.33 | 0.56 ± 0.31 |
| 30 | 1.80 ± 0.32 | 0.73 ± 0.37b | 1.00 ± 0.47bd | 0.95 ± 0.47b | 0.87 ± 0.46b | |
| Pb=0.001 | Pb=0.001, Pd=0.003 | Pb=0.001 | Pb=0.001 | |||
| 60 | 2.66 ± 0.59 | 1.01 ± 0.54b | 1.46 ± 0.70bd | 1.33 ± 0.78b | 1.37 ± 0.71b | |
| Pb=0.001 | Pb=0.001, Pd=0.001 | Pb=0.001 | Pb=0.001 | |||
| 120 | 2.42 ± 0.58 | 1.46 ± 0.91b | 2.22 ± 1.30d | 1.78 ± 1.00a | 2.02 ± 1.10d | |
| Pb=0.001 | Pd=0.001 | Pa=0.040 | Pd=0.001 | |||
| 180 | 1.60 ± 0.36 | 1.42 ± 0.82 | 2.18 ± 1.24bd | 1.82 ± 1.42 | 2.14 ± 1.06bd | |
| N.S. | Pb=0.006, Pd=00.001 | N.S. | Pb=0.007, Pd=0.001 | |||
Table 3: CP at different time points before and after the treatment.
| Control | Non-obese T2DM | Obese T2DM | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| Glucagon | 0 | 108.52 ± 9.19 | 111.46 ± 34.49 | 101.48 ± 28.90 | 117.33 ± 29.90 | 110.60 ± 36.22 |
| 30 | 118.97 ± 9.72 | 151.88 ± 54.65b | 131.93 ± 34.53ad | 153.61 ± 40.02b | 142.36 ± 39.82b | |
| Pb=0.001 | Pa=0.031, Pd=0.007 | Pb=0.001 | Pb=0.001 | |||
| 60 | 118.46 ± 9.43 | 155.89 ± 47.62b | 140.52 ± 37.16bd | 160.09 ± 39.22b | 150.89 ± 49.78b | |
| Pb=0.001 | Pb=0.001, Pd=0.009 | Pb=0.001 | Pb=0.001 | |||
| 120 | 110.32 ± 7.68 | 141.58 ± 50.67b | 126.34 ± 40.53ac | 136.39 ± 37.61b | 128.67 ± 44.22b | |
| Pb=0.004 | Pa=0.016, Pc=0.010 | Pb=0.001 | Pb=0.009 | |||
| 180 | 101.63 ± 7.33 | 120.54 ± 39.32b | 109.43 ± 32.91c | 117.55 ± 29.86b | 107.51 ± 32.34c | |
| Pb=0.001 | Pc=0.024 | Pb=0.002 | Pc=0.022 | |||
Table 4: Glucagon at different time points before and after the treatment.
After treatment, the blood glucose levels in the non-obese and obese T2DM groups were still significantly higher than that in the control group (P=0.001), but had significantly decreased compared to that before treatment (P=0.001, Table 2). The CP levels after treatment in the non-obese T2DM group were significantly lower than those in the control group at T1 (P=0.001), T2 (P=0.001), and T4 (P=0.006), but had significantly increased at T1 (P=0.003), T2 (P=0.001), T3 (P=0.001), and T4 (P=0.001) compared with that before treatment. The CP levels after treatment in the obese T2DM group were significantly lower than those in the control group at T1 (P=0.001), T2 (P=0.001), and T4 (P=0.007), but had significantly increased at T3 (P=0.001) and T4 (P=0.001, Table 3) compared with that before treatment. The glucagon levels after treatment in the non-obese T2DM group were significantly higher than those in the control group at T1 (P=0.031), T2 (P=0.001), and T3 (P=0.016). The glucagon levels after treatment in the non-obese T2DM group had significantly decreased at T1 (P=0.007), T2 (P=0.009), T3 (P=0.010), and T4 (P=0.024) compared with that before treatment. The glucagon levels after treatment in the obese T2DM group were significantly higher than those in the control group at T1 (P=0.001), T2 (P=0.001), and T3 (P=0.009). The glucagon levels in the obese T2DM group after treatment also had significantly decreased at T4 (P=0.022) compared with that before treatment (Table 4).
Discussion
This study mainly investigated the effects of insulin therapy on glucagon levels in non-obese and obese T2DM patients. In most patients with T2DM, two metabolic defects can occur: insulin resistance and/or insulin secretion deficiency. Insulin deficiency can reduce the inhibition of glucagon. Previous studies [29-31] showed that endogenous insulin may act directly on the insulin receptors of α-cell membranes and inhibit the secretion of glucagon through the PI 3-kinase/Akt signalling pathway. Alternatively, it may indirectly reduce the sensitivity of the α-cell KATP channels [32] and strengthen the γ-amino butyric acid pathway [30] to inhibit glucagon secretion. In the present study, the overall glucagon levels in the T2DM group were higher than those in the control group, which was consistent with the results of previous studies [33]. However, there were no differences in basal glucagon levels among the patients with T2DM. This may be related with the lack of significant differences in the basal insulin level, compared with the control group. Patients with T2DM who have better blood glucose control have small variations in fasting glucagon levels, but those with poor blood glucose control or ketosis have significantly higher basal glucagon levels than healthy people [7,34]. The postprandial glucagon levels in the patients with T2DM were higher than that in the control group. Postprandial glucagon levels tend to increase throughout the mixed meal test [16] or oral glucose tolerance test [4]. The increase in postprandial glucagon could be related with impaired insulin secretion, and the decrease in postprandial insulin secretion impairs the inhibitory effects on glucagon [35-38]. In the present study, patients with T2DM had a deficiency in postprandial insulin secretion, supporting the findings of previous studies.
Insulin therapy is a shared method for treating T2DM; as observed in previous studies, the present study found that the glucagon levels decreased after insulin treatment in patients with T2DM [26,27]. The results suggest that insulin treatment could reduce glucagon levels in patients with T2DM. This phenomenon is related with the direct and indirect actions of endogenous insulin on the islet α-cells. The administration of exogenous insulin prevents the inhibitory action of endogenous insulin on glucagon secretion by α-cells. Similar to previous studies, in the present study, after treatment using exogenous insulin, endogenous insulin secretion was restored to some extent, and this recovery was reflected by the elevated level of CP [39,40]. The recovery of endogenous insulin may enhance the inhibition of glucagon secretion via the PI 3-kinase/Akt and γ-amino butyric acid pathways, as already discussed. Certain studies [32,41] also reported that after treatment using exogenous insulin, insulin secreted by in vivo β-cells increased, and this increase may cause the secretion of Zn2+, which has inhibitory effects on glucagon.
The relationship between glucagon and obesity is a popular research topic. However, consensus regarding this relationship has not yet been reached. An increase in glucagon levels might be associated with obesity-related insulin resistance [42,43]. In addition, obese people with normal glucose tolerance have higher glucagon levels [22,44]. Changes in levels of leptin [45,46] and inflammatory cytokines [22] associated with obesity-related insulin resistance could elevate glucagon levels. The increase in gastric inhibitory polypeptide levels in obese people with normal glucose tolerance and in patients with T2DM after an oral glucose tolerance test might be related with increased postprandial glucagon levels [17,34]. In obese patients with T2DM, findings regarding glucagon levels differ. In one study, the overall glucagon levels in obese patients with T2DM were higher than those in non-obese patients with T2DM [16]. In another study, there were no differences in the basal and post-mixed meal test glucagon levels between obese and non-obese individuals with T2DM [10]. In the present study, both non-obese and obese patients with T2DM had higher glucagon levels than the control group. The absolute basal and postprandial glucagon levels in the obese patients with T2DM at all the time intervals were slightly higher than those in the non-obese patients with T2DM, but the differences were not statistically significant. The results of the present study may be related with the similar degrees of obesity (BMI of 26.82 kg/m2 vs. 21.80 kg/m2).
The present study further analysed the effects of insulin therapy on glucagon levels in non-obese and obese patients with T2DM. After treatment, the glucagon levels overall and at different postprandial time intervals in non-obese patients with T2DM decreased significantly; however, in the obese T2DM group, these levels did not decrease as much and were only significantly different at T4. This decrease in the non-obese patients with T2DM may be associated with the noticeable recovery of endogenous insulin in non-obese patients with T2DM. CP levels reflect endogenous insulin levels. After treatment, CP levels in non-obese patients with T2DM increased at all of the postprandial time intervals. The increased CP levels suggest that the functions of in vivo β-cells recovered, and glucose toxicity decreased to some extent. However, insulin therapy did not significantly reduce the glucagon level in obese patients with T2DM, which may be related with insufficient recovery of the endogenous insulin secretion [47]. In the present study, simple insulin therapy did not significantly improve endogenous insulin secretion, and the postprandial CP levels after treatment only showed statistically significant improvements at T3 and T4.
In conclusion, non-obese and obese nd-T2DM patients have lower insulin levels and higher glucagon levels than people without T2DM. Insulin therapy can decrease glucagon levels. A greater decrease in glucagon levels was observed in nonobese patients with T2DM after treatment than in obese patients with T2DM; the decrease was not statistically significant for the obese patients with T2DM. After treatment, CP levels increased. This increase mainly occurred in nonobese patients with T2DM, and the changes for obese patients with T2DM were not statistically significant. New treatments or programs that decrease glucagon levels and increase CP levels in obese patients with T2DM need to be investigated further.
Acknowledgements
Special thanks should be given to the Third Affiliated Hospital of Anhui Medical University.
Conflicts of Interest
All of the authors declare that they have no conflicts of interest regarding this paper.
References
- He XS, Wang ZX, Zhu YZ, Wang N, Hu X. Hyperactivation of working memory-related brain circuits in newly diagnosed middle-aged type 2 diabetics. Acta Diabetol 2015; 52: 133-142.
- Shah P, Basu A, Basu R, Rizza R. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol 1999; 277: E283-290.
- Knop FK, Vilsboll T, Hojberg PV, Larsen S, Madsbad S, Volund A, Holst JJ, Krarup T. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 2007; 56: 1951-1959.
- Knop FK, Vilsboll T, Madsbad S, Holst JJ, Krarup T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia 2007; 50: 797-805.
- Shah P, Vella A, Basu A, Basu R, Schwenk WF. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 2000; 85: 4053-4059.
- Bagger JI, Knop FK, Lund A, Holst JJ, Vilsboll T. Glucagon responses to increasing oral loads of glucose and corresponding isoglycaemic intravenous glucose infusions in patients with type 2 diabetes and healthy individuals. Diabetologia 2014; 57: 1720-1725.
- Ohneda A, Watanabe K, Horigome K, Sakai T, Kai Y. Abnormal response of pancreatic glucagon to glycemic changes in diabetes mellitus. J Clin Endocrinol Metab 1978; 46: 504-510.
- Arner P, Pollare T, Lithell H. Different aetiologies of type 2 (non-insulin-dependent) diabetes mellitus in obese and non-obese subjects. Diabetologia 1991; 34: 483-487.
- Unger RH. The milieu interieur and the islets of Langerhans. Diabetologia 1981; 20: 1-11.
- Basu R, Chandramouli V, Dicke B, Landau B, Rizza R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes 2005; 54: 1942-1948.
- Watts M, Sherman A. Modeling the pancreatic α-cell: dual mechanisms of glucose suppression of glucagon secretion. Biophys J 2014; 106: 741-751.
- Lund A, Bagger JI, Christensen M, Knop FK, Vilsboll T. Glucagon and type 2 diabetes: the return of the alpha cell. Curr Diab Rep 2014; 14: 555.
- Godoy-Matos AF. The role of glucagon on type 2 diabetes at a glance. Diabetol Metab Syndr 2014; 6: 91.
- Ahren B. Glucagon-early breakthroughs and recent discoveries. Peptides 2015; 67: 74-81.
- Gosmain Y, Masson MH, Philippe J. Glucagon: the renewal of an old hormone in the pathophysiology of diabetes. J Diabetes 2013; 5: 102-109.
- Kozawa J, Okita K, Iwahashi H, Yamagata K, Imagawa A, Shimomura I. Early postprandial glucagon surge affects postprandial glucose levels in obese and non-obese patients with type 2 diabetes. Endocr J 2013; 60: 813-818.
- Knop FK, Aaboe K, Vilsbøll T, Volund A, Holst JJ, Krarup T, Madsbad S. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes ObesMetab 2012; 14: 500-510.
- Abdul-Ghani M, Defronzo RA. Fasting hyperglycaemia impairs glucose- but not insulin-mediated suppression of glucagon secretion. J ClinEndocrinolMetab 2007; 92: 1778-1784.
- Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 2007; 28: 84-116.
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 2006; 103: 2334-2339.
- Cooperberg BA, Cryer PE. Beta-cell-mediated signalling predominates over direct alpha-cell signalling in the regulation of glucagon secretion in humans. Diabetes Care 2009; 32: 2275-2280.
- Ellingsgaard H, Ehses JA, Hammar EB, Van Lommel L, Quintens R. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci USA 2008; 105: 13163-13168.
- Barnes TM, Otero YF, Elliott AD, Locke AD, Malabanan CM, Coldren AG, Brissova M, Piston DW, McGuinness OP. Interleukin-6 amplifies glucagon secretion: coordinated control via the brain and pancreas. Am J PhysiolEndocrinolMetab 2014; 307: E896-E905.
- Kawasaki F1, Matsuda M, Kanda Y, Inoue H, Kaku K. Structural and functional analysis of pancreatic islets preserved by pioglitazone in db/db mice. Am J Physiol Endocrinol Metab 2005; 288: E510-518.
- Hamamoto S, Kanda Y, Shimoda M, Tatsumi F, Kohara K, Tawaramoto K, Hashiramoto M, Kaku K. Vildagliptin preserves the mass and function of pancreatic beta cells via the developmental regulation and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetes ObesMetab2013; 15: 153-163.
- Gossain VV, Rovner DR. Pancreatic glucagon: possible implications of the hyperglycemic hormone in diabetes control. Postgrad Med 1982; 72: 87-88, 91-93, 96.
- Moon JS, Won KC. Pancreatic α-cell dysfunction in type 2 diabetes: old kids on the block. Diabetes Metab J 2015; 39: 1-9.
- Menge BA, Gruber L, Jorgensen SM, Deacon CF, Schmidt WE, Veldhuis JD, Holst JJ, Meier JJ. Loss of inverse relationship between pulsatile insulin and glucagon secretion in patients with type 2 diabetes. Diabetes 2011; 60: 2160-2168.
- Velloso LA, Carneiro EM, Crepaldi SC, Boschero AC, Saad MJ. Glucose- and insulin-induced phosphorylation of the insulin receptor and its primary substrates IRS-1 and IRS-2 in rat pancreatic islets. FEBS Lett 1995; 377: 353-357.
- Xu E, Kumar M, Zhang Y, Ju W, Obata T. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab 2006; 3: 47-58.
- Albury-Warren TM, Pandey V, Spinel LP, Masternak MM, Altomare DA. Prediabetes linked to excess glucagon in transgenic mice with pancreatic active AKT1. J Endocrinol 2016; 228: 49-59.
- Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 2005; 54: 1808-1815.
- Bagger JI, Knop FK, Lund A, Holst JJ, Vilsboll T. Glucagon responses to increasing oral loads of glucose and corresponding isoglycaemic intravenous glucose infusions in patients with type 2 diabetes and healthy individuals. Diabetologia 2014; 57: 1720-1725.
- DAlessio D. The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes Metab 2011; 13: 126-132.
- Hope KM, Tran PO, Zhou H, Oseid E, Leroy E. Regulation of alpha-cell function by the beta-cell in isolated human and rat islets deprived of glucose: the switch-off hypothesis. Diabetes 2004; 53: 1488-1495.
- Hamaguchi T, Fukushima H, Uehara M, Wada S, Shirotani T, Kishikawa H, Ichinose K, Yamaguchi K, Shichiri M. Abnormal glucagon response to arginine and its normalization in obese hyperinsulinaemic patients with glucose intolerance: importance of insulin action on pancreatic alpha cells. Diabetologia 1991; 34: 801-806.
- Cooperberg BA, Cryer PE. Insulin reciprocally regulates glucagon secretion in humans. Diabetes 2010; 59: 2936-2940.
- Cryer PE. Minireview: Glucagon in the pathogenesis of hypoglycaemia and hyperglycaemia in diabetes. Endocrinol 2012; 153: 1039-1048.
- Li Y, Xu W, Liao Z, Yao B, Chen X. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care 2004; 27: 2597-2602.
- Thompson JS, Duckworth WC. Insulin pumps and glucose regulation. World J Surg 2001; 25: 523-526.
- Solomou A, Meur G, Bellomo E, Hodson DJ, Tomas A, Li SM, Philippe E, Herrera PL, Magnan C, Rutter GA. The zinc transporter Slc30a8/ZnT8 is required in a subpopulation of pancreatic alpha-cells for hypoglycemia-induced glucagon secretion. J BiolChem 2015; 290: 21432-21442.
- Ferrannini E, Muscelli E, Natali A, Gabriel R, Mitrakou A, Flyvbjerg A, Golay A, Hojlund K. Relationship between insulin sensitivity and Cardiovascular Disease Risk (RISC) Project Investigators. Association of fasting glucagon and proinsulin concentrations with insulin resistance. Diabetologia 2007; 50: 2342-2347.
- Suppli MP, Lund A, Bagger JI, Vilsboll T, Knop FK. Involvement of steatosis-induced glucagon resistance in hyperglucagonaemia. Med Hypotheses 2016; 86: 100-103.
- Starke AA, Erhardt G, Berger M, Zimmermann H. Elevated pancreatic glucagon in obesity. Diabetes 1984; 33: 277-280.
- Chen L, Philippe J, Unger RH. Glucagon responses of isolated alpha cells to glucose, insulin, somatostatin, and leptin. EndocrPract 2011; 17: 819-825.
- Reno CM, Ding Y, Sherwin R. Leptin acts in the brain to influence hypoglycaemic counterregulation: disparate effects of acute and recurrent hypoglycaemia on glucagon release. Am J PhysiolEndocrinolMetab 2015; 309: E960-E967.
- Park SW, Ihm SH, Yoo HJ, Park JY, Lee KU. Differential effects of ambient blood glucose level and degree of obesity on basal serum C-peptide level and the C-peptide response to glucose and glucagon in non-insulin-dependent diabetes mellitus. Diabetes Res ClinPract 1997; 37: 165-171.