- Biomedical Research (2013) Volume 24, Issue 3
Effects of Hypericum perforatum and Hippophae rhamnoides extracts on indomethacin-induced gastric oxidative stress in rats.
Mehmet Ibrahim Turan1*, Habib Bilen2, Ismail Demiryilmaz3, Fatma Betul Ozgeris4 , Huseyin BaykalJ5, Murat Turkoglu6 , Halis Suleyman 71Department of Pediatrics, Faculty of Medicine, Ataturk University, Erzurum, Turkey
2Department of Internal Medicine, Faculty of Medicine, Ataturk University, Erzurum, Turkey
3Department of General Surgery, Ibni Sina Hospital, Kayseri, Turkey
4Department of Biochemistry, Faculty of Medicine, Ataturk University, Erzurum, Turkey
5Recep Tayyip Erdoğan University Pazar Vocationaly High School. Plant and Animal Breeding Department, Medicinal and Aromatic Plants Programme, Rize, Turkey
6Biota laboratories, 34785, Sancaktepe, Istanbul, Turkey
7Department of Pharmacology, Faculty of Medicine, Ataturk University, Erzurum, Turkey Research was conducted in Pharmacology Department’s laboratories in Ataturk University, Erzurum, Turkey
- *Corresponding Author:
- Mehmet Ibrahim Turan
Department of Pediatric Neurology
Faculty of Medicine
Ataturk University
Erzurum, Turkey
Accepted date: March 16 2013
Citation: Turan MI, Bilen H, Demiryilmaz I, Ozgeris FB, Baykal H, Turkoglu M, Suleyman H. Biomedical Effects of Hypericum perforatum and Hippophae rhamnoides extracts on indomethacin-induced gastric oxidative stress in rats. Biomed Res- India; 2013; 24 (3): 314-319.
Abstract
We investigate the effects of Hypericum perforatum (HP) and Hippophae rhamnoides (HR) extracts on indomethacin-induced gastric injury. Rats were divided into five groups each containing 10 animals. The first group received HP extract, the second group received HR, and the third group received a combination of HP:HR extract at a ratio of 60:40. Fourth group had indomethacin (control group). Fifth group was untreated healthy group. First four groups of animals were gavaged indomethacin and the degree of gastric injury was recorded. Glutathione (GSH), malondialdehyde (MDA) and DNA injury products from the gastric tissue were measured. In the indomethacin-treated control group, the ulcer area was 78.8±2.5/mm2 and was significantly larger than the animals of HP, HR, HP+HR groups. When the groups were compared with the indomethacin-treated control group for GSH, MDA and DNA injury, the differences between all the groups were statistically significant. The mixture of HP and HR extracts showed maximum protective effects on indomethacin-induced gastric injury.
Keywords
Hypericum, Hippophae, Rat, Oxidative stress
Introduction
Gastric ulcers are multifactorial and recurrent disorders that are frequently seen today. Gastric lesion results as a consequence of impaired balance between gastroprotective factors such as mucus, bicarbonate and prostaglandins, and gastro-destructive substances [1]. Nonsteroidal anti-inflammatory drugs (NSAIDs) damage the structure of these protective factors and result in negative effects on the stomach [2]. In gastric injury, it has been shown that NSAIDs cause oxidative injury by the effect of reactive oxygen products on the gastric mucosa [3, 4]. Many drugs and plants have been investigated for their healing effects on gastric ulcer [5]. In spite of many studies performed, there is no definitive treatment for gastric ulcer. In this study, centaury oil extracts known as Hypericum perforatum (HP), investigated for its effects on gastric ulcer, is obtained from the St John’s wort plant. HP, which belongs to the Clusiaceae family, is found in the natural vegetation of Europe and Asia. This plant contains flavonoid, tannin, carotenoid, choline and various amino acids [6, 7]. There are studies investigating the effects of plant extracts on oxidative stress [8, 9]. As a part of conventional treatment, they have been used in the management of different diseases in many different cultures. Although its most widely known effect is that on mild or moderate depression, the antipyretic, antispasmodic, analgesic and antimicrobial effects have also been reported. Its therapeutic effect has been demonstrated in neurological disorders such as migraine, bronchial and urogenital system inflammations, some skin diseases and ulcer [10-13].
Another oil extract investigated in the study was sea buckthorn oil, obtained from the Hippophae rhamnoides plant. Hippophae rhamnoides L. (HR) is a member of the Eleagnaceae family [14]. This plant comprises carotenoid (α,β,γ), riboflavin, Vitamin C, tocopherole, tocotrienol, folic acid and tannin [15, 16]. The antioxidant, antiulcerogenic, radioprotective, antitoxic, anticoagulant and antimicrobial effects of HR has been demonstrated [17]. Many experimental studies showing its antioxidant effects have been performed [18-20].
Although the extracts obtained from the HP and HR plants used in this study have been previously reported to have positive effects on gastric ulcer, the effects of using certain amounts of both extracts on gastric ulcer have not been investigated. Therefore, the effects of HP, HR and these plants’ mixture on indomethacin-induced gastric oxidative stress in rats were investigated.
Material and Method
Experimental animals
Fifty Wistar albino male rats weighing between 225 and 235 grams were used in this study. The animals were obtained from Atatürk University Medical Experimental Research and Practice Center. The animals were kept and fed ad libitum in groups before the experiments. They were divided into five groups with 10 rats in each.
Chemical Substances
The HP and HR plants extracts were provided by Biota Lab. (Bioxcin), Istanbul and Indomethacin was obtained from Deva Holding A.S., Istanbul.
Indomethacin Ulcer Test
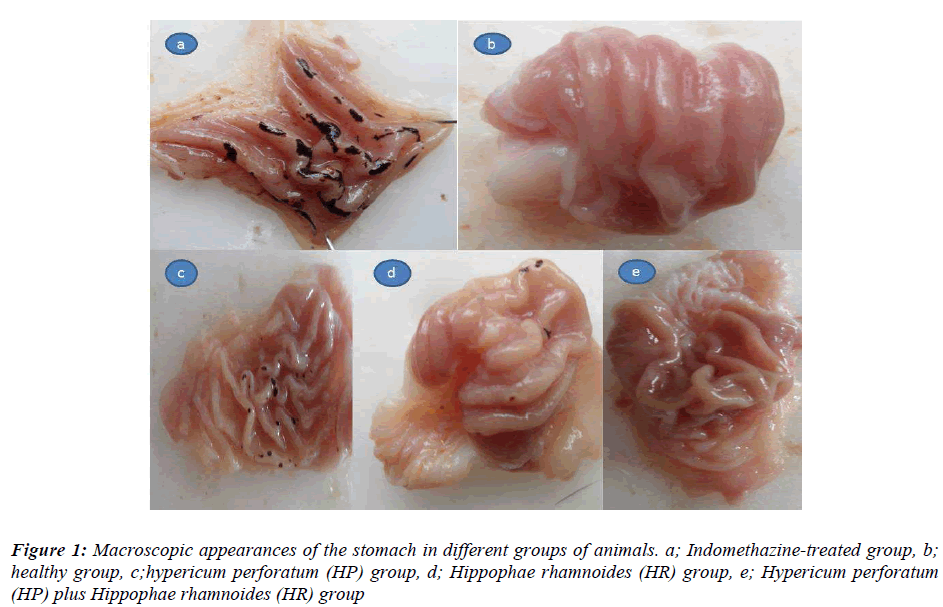
In this experiment, the anti-ulcer activities of HP and HR extracts were investigated in the indomethacin-induced gastric ulcer model in rats [21]. After 24 hours of starvation, the groups of rats received HP oil of 1 ml/kg BW (HP group), HR oil of 1 mg/kg BW (HR group), HP+HR oil of a mixture at a ratio of 60/40 [0.6/0.4 ml/kg BW] (HP+HR group) by gavaging. The control group received distilled water of equal volume as solvent (Indomethacin group). Five minutes following administration of medications, all the groups of rats were gavaged indomethacin at a dose of 25 mg/kg BW. The healthy group did not receive any medications. Six hours following administration of indomethacin, the animals were sacrificed by decapitation. Stomachs of the animals were extracted and the ulcer foci on the gastric surface were assessed macroscopically. The width of the ulcer area on the gastric surface was measured on a paper with mm2 scale. The anti-ulcer activities of sea buckthorn and centaury oil were assessed by comparing with the control group. Glutathione (GSH), malondialdehyde (MDA) and the degree of cellular DNA damage were measured in gastric tissue samples.
Biochemical analysis of gastric tissue
From each of the stomach, 0.2 mg of whole gastric tissue was weighed, homogenized in ice with 2-mL buffers (consisting of 0.5% HDTMAB [0.5% hexa desil tri methyl ammonium bromide] at pH: 6 potassium phosphate buffer for myeloperoxidase analyze, consisting of 1.15% potassium chloride solution for thio barbituric acid reactions (TBARS) analysis and at pH: 10.5 phosphate buffer for the superoxide dismutase, total glutathione analysis. The preparation was then centrifuged at 4 °C, 10.000 rpm for 15 minutes. The supernatant part was used for analysis.
Total glutathione (tGSH) analysis
The amount of GSH in the total homogenate was measured according to the method of Sedlak and Lindsay with some modifications [22].
Determination of lipid peroksidation or Malondialdehyde (MDA)
The concentrations of gastric tissue lipid peroxidation were determined by using the TBARS, a modified version of the method used by Nabavi [23].
Isolation of DNA from gastric tissue
DNA isolated was made using modified method of Shigenega et al. [24].
DNA hydrolysis with formic acid
Approximately 50 mg of DNA was hydrolyzed with 0.5 mL of formic acid (60%, vol/vol) for 45 minutes at 150 °C [25]. The tubes were allowed to cool. The contents were then transferred to Pierce microvials (Sigma Co. Munich, Germany), covered with Kleenex tissues (Kimberly-Clark, USA) cut to size (secured in place using a rubber band), and cooled in liquid nitrogen. Formic acid was then removed by freeze-drying. Before analysis by high-performance liquid chromatography (HPLC), they were redissolved in the eluent (final volume 200 μL) [26, 27].
Measurement of 8-hydroxy-2 deoxyguanine (8-OH Gua) with high performance liquid chromatography (HPLC) system
The amount of 8-OH gua and guanine (Gua) was measured by using a HPLC system equipped with an electrochemical detector (HP Agilent 1100 module series, E.C.D. HP 1049 A), as described previously by Kaur and Floyd [25, 28]. The 8-OH gua levels were expressed as the number of 8-OH gua molecules/105 Gua molecules [29].
Statistical Analysis
The experiment results were expressed as "mean ± standart error of the mean " (X±SEM). The significance of the difference between the groups was determined using the one-way ANOVA test. The Fisher’s post-hoc Scheffe test was performed afterwards. All of the statistical processes were carried out using the “Statistical Package for Social Sciences 18.0 (Armonk, NY, USA) software” and p value of ≤ 0.001 was accepted as significant.
Results
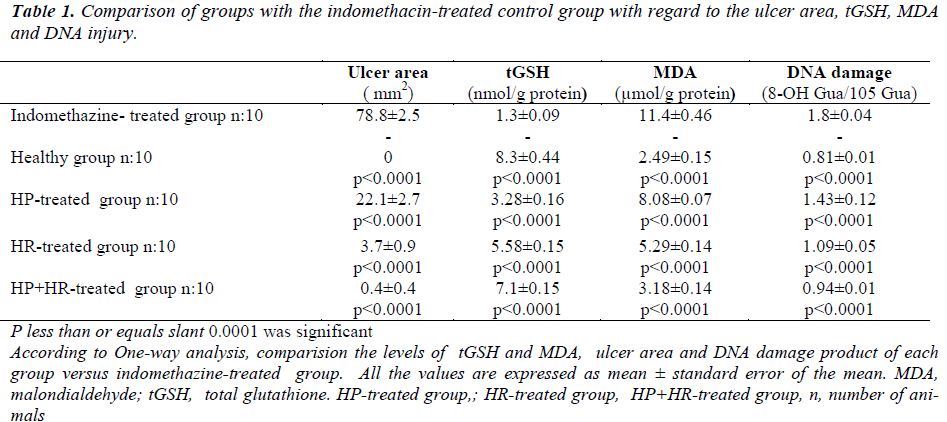
The macroscopic appearances of all groups have been presented in Figure 1. As seen in Table 1, the ulcer area in the indomethacin-treated control group was 78.8±2.5/mm2; this was significantly different from the HP, HR, and HP+HR-treated groups. When the HP+HRtreated group was compared with the HP and HR-treated groups, a significant difference with the HP group was recorded, while the difference was found to be insignificant with the HR-treated group (p = 0.7).
Table 1 demonstrates that the comparison of groups with the indomethacin-treated control group with respect to tGSH, MDA and DNA damage revealed significant differences between the groups. The HP and the HR-treated groups showed significant differences with the HP+HRtreated group (p<0.0001).
Discussion
In this study, the effects of HP, HR and a mixture of HP and HR extracts at a ratio of 60:40 were investigated on indomethacin-induced gastric injury and oxidative stress. Experimental studies showed that indomethacin causes gastric injury [30]. In the present study, indomethacininduced gastric injury was found to be protected best by the mixture of HP+HR extract. Present finding confirms the findings of others [11, 17]. In indomethacin-induced gastric injury, it has been shown that the levels of ROS exceed the concentration of antioxidant [31]. In this study, increased tissue MDA suggests an increase in free oxygen radicals. The most important and harmful effect that free radicals initiate in the cell is lipid peroxidation [32, 33]. MDA causes further destruction in the cells [34]. tGSH is involved in cell protection against oxidative injury and metabolic processes of many endogenous compounds such as estrogen, prostaglandins and leukotrienes [35]. As an antioxidant, tGSH reacts with peroxides and free radicals and converts them into harmless products. tGSH protects the cell from the oxidative injury that the free radicals may cause [36]. tGSH also prevents –SH groups in proteins from oxidation by keeping them in the reduced form [37, 38] . It has been shown that in the injured tissues, amount of MDA increases compared to the levels of tGSH [39].
The DNA damage product, 8-OH Gua, is related with increased oxidative stress [40]. In the indomethacininduced gastric ulcer model, oxidative stress caused by reactive oxygen radicals enhances mucosal injury in gastric epithelial tissue [30]. 8-OH Gua is a very sensitive marker of DNA damage caused by reactive oxygen radicals [41].
When compared with the control group, the closest results to those of the healthy group with respect to GSH, MDA and DNA damage products were obtained with the HP+HR-treated group. Results obtained with the HRtreated group were more or less similar to that of the healthy subjects than the HP-treated group, which means that the antioxidant effect of HR extract was higher than that of the HP. In spite of the fact that the HP plant possesses hypericin and its derivatives, hyperforin, flavonoids (hyperoside, rutin, quercetin vs), catechin, epicatechin, procyanidin B2, amino acid derivatives (melatonin, GABA), and fatty acids, the antioxidant effects of molecules like carotenoid (α,β,γ), riboflavin, vitamin C, tocopherol, folic acid and tannin in the HR plant seem to be more potent as anti-oxidant [15, 42, 43].
In conclusion, combined extracts of HP and HR compared to an individual extract of HP or HR was found to provide best protective effect on indomethacin-induced gastric injury. However, further research on the molecular basis of action of these herbal extracts in protecting gastric ulceration is very much needed before proposing these herbal mixture as an alternative therapy.
References
- Tytgat GN. Etiopathogenetic principles and peptic ulcer disease classification. Dig Dis 2011; 29: 454-458.
- Belaiche J, Burette A, De Vos M, Louis E, Huybrechts M, Deltenre M. Observational survey of NSAIDrelated upper gastro-intestinal adverse events in Belgium. Acta Gastroenterol Belg 2002; 65: 65-73.
- Chattopadhyay I, Bandyopadhyay U, Biswas K, Maity P, Banerjee RK. Indomethacin inactivates gastric peroxidase to induce reactive-oxygen-mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic Biol Med 2006; 40: 1397-1408.
- Das D, Bandyopadhyay D, Bhattacharjee M, Banerjee RK. Hydroxyl radical is the major causative factor instress-induced gastric ulceration. Free Radic Biol Med 1997, 23:8-218.
- Hiruma-Lima CA, Gracioso JS, Bighetti EJ, Grassi- Kassisse DM, Nunes DS, Brito AR. Effect of essential oil obtained from Croton cajucara Benth. on gastric ulcer healing and protective factors of the gastric mucosa. Phytomedicine 2002; 9: 523-529.
- Greeson JM, Sanford B, Monti DA. St. John's wort (Hypericum perforatum): a review of the current pharmacological, toxicological, and clinical literature. Psychopharmacol (Berl) 2001; 153: 402-414.
- Bombardelli E, Morazzoni P. Hypericum perforatum. Fitoterapia 1995; 66: 43-68.
- Kumar A, Garg R, Prakash AK. Effect of St. John's Wort (Hypericum perforatum) treatment on restraintstress-induced behavioral and biochemical alteration in mice. BMC Complement Altern Med 2010; 10: 18.
- Ekin S, Oto G, Yardim Y, Levent A, Ozgokce F, Kusman T. Protective effect of Hypericum perforatumL. on serum and hair trace elements in rats 7,12-dimethylbenz[a]anthracene-induced oxidative stress.Environ Toxicol Pharmacol 2012; 33(3): 440-445.
- Hammer KD, Hillwig ML, Solco AK, Dixon PM, Delate K, Murphy PA, Wurtele ES, Birt DF. Inhibitionof prostaglandin E(2) production by anti-inflammatory hypericum perforatum extracts and constituents in RAW264.7 Mouse Macrophage Cells. J Agric Food Chem 2007; 55(18): 7323-7331.
- Zdunic G, Godevac D, Milenkovic M, Vucicevic D, Savikin K, Menkovic N, Petrovic S. Evaluation of Hypericum perforatum oil extracts for an antiinflammatory and gastroprotective activity in rats. Phytother Res 2009; 23(11): 1559-1564.
- Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D. St John's wort for depression--an overview and meta-analysis of randomised clinical trials. Bmj 1996; 313(7052):253-258.
- WHO: Monographs on Selected Medicinal Plants, Herba Hyperici. In. Edited by Organization WH, vol. 2.Geneva 2002; 149-171.
- Rousi A. The genus Hippophue L. A taxonomic study. Ann Bot Fennici 1971; 8: 177-227.
- Suleyman H, Demirezer LO, Buyukokuroglu ME, Akcay MF, Gepdiremen A, Banoglu ZN, Gocer F.Antiulcerogenic effect of Hippophae rhamnoides L. Phytother Res 2001; 15(7): 625-627.
- Andersson SC, Rumpunen K, Johansson E, Olsson ME. Tocopherols and tocotrienols in sea buckthorn(Hippophae rhamnoides L.) berries during ripening. J Agric Food Chem 2008; 56(15): 6701-6706.
- Guliyev VB, Gul M, Yildirim A. Hippophae rhamnoides L.: chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effects. J Chromatogr B Analyt Technol Biomed Life Sci 2004; 812(1-2): 291-307.
- Suleyman H, Gumustekin K, Taysi S, Keles S, Oztasan N, Aktas O, Altinkaynak K, Timur H, Akcay F, Akar S, Dane S, Gul M. Beneficial effects of Hippophae rhamnoides L. on nicotine induced oxidative stress in rat blood compared with vitamin E. Biol Pharm Bull 2002; 25(9): 1133-1136.
- Geetha S, Sai Ram M, Mongia SS, Singh V, Ilavazhagan G, Sawhney RC. Evaluation of antioxidant activity of leaf extract of Seabuckthorn (Hippophae rhamnoides L.) on chromium(VI) induced oxidative stress in albino rats. J Ethnopharmacol 2003; 87(2-3): 247-251.
- Geetha S, Sai Ram M, Singh V, Ilavazhagan G, Sawhney RC. Anti-oxidant and immunomodulatory properties of seabuckthorn (Hippophae rhamnoides)-- an in vitro study. J Ethnopharmacol 2002; 79(3):373- 378.
- Guidobono F, Pagani F, Ticozzi C, Sibilia V, Pecile A,Netti C. Protection by amylin of gastric erosions induced by indomethacin or ethanol in rats. Br J Pharmacol 1997; 120(4): 581-586.
- Sedlak J, Lindsay RH. Estimation of total, proteinbound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Analytical biochemistry 1968; 25(1):192-205.
- Nabavi SF, Nabavi SM, Abolhasani F, Moghaddam AH, Eslami S. Cytoprotective effects of curcumin on sodium fluoride-induced intoxication in rat erythrocytes. Bulletin of environmental contamination and toxicology 2012; 88(3): 486-490.
- Shigenaga MK, Aboujaoude EN, Chen Q, Ames BN. Assays of oxidative DNA damage biomarkers 8-oxo-2'-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high-performance liquid chromatography with electrochemical detection. Methods in enzymology 1994; 234: 16-33.
- Kaur H, Halliwell B. Measurement of oxidized and methylated DNA bases by HPLC with electrochemicaldetection. The Biochemical journal 1996; 318 (Pt 1): 21-23.
- Senturker S, Dizdaroglu M. The effect of experimental conditions on the levels of oxidatively modified bases in DNA as measured by gas chromatography-mass spectrometry: how many modified bases are involved? Prepurification or not? Free radical biology & medicine 1999; 27(3-4): 370-380.
- Mosca F, Fattorini D, Bompadre S, Littarru GP. Assay of coenzyme Q(10) in plasma by a single dilution step. Analytical biochemistry 2002, 305(1): 49-54.
- Floyd RA, Watson JJ, Wong PK, Altmiller DH, Rickard RC. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free radical research communications 1986; 1(3): 163-172.
- Asami S, Hirano T, Yamaguchi R, Tomioka Y, Itoh H, Kasai H. Increase of a type of oxidative DNA damage, 8-hydroxyguanine, and its repair activity in human leukocytes by cigarette smoking. Cancer research 1996; 56(11): 2546-2549.
- Kisaoglu A, Ozogul B, Cetin N, Suleyman B, Atamanalp SS, Akcay F, Suleyman H. The Role of Alpha-2 drenergic Receptors in the Anti-ulcerative Activity of Famotidine and Omeprazole in Rats and its Relationship with Oxidant-antioxidant Parameters.International Journal of Pharmacology 2011; 7(6):682- 689.
- Naito Y, Yoshikawa T, Yoshida N, Kondo M. Role of oxygen radical and lipid peroxidation in indomethacininduced gastric mucosal injury. Dig Dis Sci 1998; 43(9 Suppl):30S-34S.
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res1998; 39(8): 1529-1542.
- Abd El-Kader MA, Ali MM, El-Sammad NM, El- Shaer MA. Antiulcer Effects of Alpha Lipoic Acid on Gastric Acid Secretion and Mucosal Defense Factors in Rats. Asian Journal of Biochemistry 2011; 6(6): 426- 438.
- Slater TF. Free-radical mechanisms in tissue injury. Biochem J 1984; 222(1): 1-15.
- Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applicationsin research and therapy. Pharmacol Ther 1991; 51(2): 155-194.
- Simona J, Joško O, Janja M. Molecular impact of glutathione peroxidases in antioxidant processes. Biochemia Medica 2008; 18(2): 162-174.
- Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003; 189(1-2):41-54.
- Mahmoud AH, Wahba HE, Ebrahim AY. Study of Some Antioxidant Parameters in Mice Livers Affected with Urtica pilulifera Extracts. Asian Journal of Biochemistry 2006; 1(1):67-74.
- Rodrigues MA, Rodrigues JL, Martins NM, Barbosa F, Curti C, Santos NA, Santos AC. Carvedilol protectsagainst cisplatin-induced oxidative stress, redox state unbalance and apoptosis in rat kidney mitochondria. Chem Biol Interact 2011; 89(1-2): 45-51.
- Takane M, Sugano N, Iwasaki H, Iwano Y, Shimizu N, Ito K. New biomarker evidence of oxidative DNA damage in whole saliva from clinically healthy and periodontally diseased individuals. J Periodontol 2002; 73(5): 551-554.
- Halliwell B, Aruoma OI. DNA damage by oxygenderived species. Its mechanism and measurement in mammalian systems. FEBS Lett 1991; 281(1-2): 9-19.
- Butterweck V, Jurgenliemk G, Nahrstedt A, Winterhoff H. Flavonoids from Hypericum perforatum showantidepressant activity in the forced swimming test.Planta Med 2000; 66(1): 3-6.
- Laakmann G, Schule C, Baghai T, Kieser M. St. John's wort in mild to moderate depression: the relevance of hyperforin for the clinical efficacy. Pharmacopsychiatry 1998; 31 Suppl 1: 54-59.