- Biomedical Research (2012) Volume 23, Issue 3
Effects of DMSO (Dimethyl sulfoxide) free cryopreservation with program freezing using a magnetic field on periodontal ligament cells and dental pulp tissues.
T. Kawata1*, S. Abedini1, M. Kaku1, H. Koseki1, S. Kojima1, H. Sumi1, M. Motokawa1, T. Fujita1, J. Ohtani1, N. Ohwada2, K. Tanne11Department of Orthodontics and Craniofacial Developmental Biology, Hiroshima University, Graduate School of Biomedical Science, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8553, Japan
2ABI Corporation Ltd., 501 Toukatsu Techno Plaza, 5-4-6 Kashiwanoha, Kashiwa-City, Chiba 277-0882, Japan
- *Corresponding Author:
- T. Kawata
Department of Orthodontics and Craniofacial Developmental Biolog
Hiroshima University
Graduate School of Biomedical Sciences
1-2-3 Kasumi, Minami-ku
Hiroshima 734-8553, Japan
Tel: 082-257-5686
Fax: 082-257-5687
E-mail: mkaku @hiroshima-u.ac.jp
Accepted date: May 02 2012
Abstract
The purpose of this study was to investigate the effects of short-term cryopreservation on the root of an incomplete human tooth with periodontal ligament cells (PDL) and dental pulp tissues. Extracted teeth were cryopreserved for three months using a programmed freezer combined with a magnetic field, known as Cells Alive System “CAS”. PDL regeneration and appropriate apexogenesis after transplanting a magnetically cryopreserved immature tooth were clinically confirmed. These findings demonstrate that teeth banking with the use of a magnetic field programmed freezer can be available for future autotransplantation as a treatment modality for replacing missing teeth. PDL regeneration and appropriate apexogenesis after transplanting a magnetically cryopreserved immature tooth were clinically confirmed. These findings demonstrate that teeth banking with the use of a magnetic field programmed freezer can be available for future autotransplantation as a treatment modality for replacing missing teeth. Results indicate that short-term cryopreservation with the use of a CAS freezer does not affect the rate of recovery or characteristics of PDL cells.
Keywords
Cryopreservation, root of an incomplete human tooth, autotransplantation, PDL cell, pulp cell, magnetic field
Introduction
Autotransplantation of teeth with a long dental care history and some influential advantages such as maintaining bone volume, PDL regeneration ability, and allowing dentofacial development has always been a treatment modality for substituting missing teeth [1, 2]. However, in situations where extracted healthy tooth immediate transplantation could not be attained, autotransplantation with the use of a teeth bank adds a new dimension to treatment planning. In this aspect, it is compulsory to preserve living cells and tissues in a structurally-intact status and, more importantly, to prevent intracellular ice formation. The establishment of a newly-developed freezer using a magnetic field called “Cells Alive System” (CAS) has been used for cryopreserving food, for achieving the goal of proper freezing and maintaining original fresh tastes, and more recently for teeth, where cell damage caused by intracellular ice formation could be prevented. The magnetic field vibration function prohibits water molecules from creating clusters during the freezing process [3]. It is generally known that appropriate PDL regeneration after autotransplantation can have a direct affect on enhancing the transplantation success rate and improving prognosis [4-6]. Moreover, based on the above-mentioned clinical findings, a CAS freezer may also be helpful in the survival of PDL cells after cryopreservation. In our previous study, the optimal conditions (15 minutes hold-time at - 5℃ followed by -30℃ plunging temperature and 0.01 mT magnetic field intensity) needed for survival and viability of PDL cells using CAS freezers were determined [7]. After the program freezing procedure, teeth were transferred to a -150℃ deep freezer (MDF-11561; Snyo, Osaka, Japan) for three months. Other studies regarding the effects of cryopreservation with normal freezers on PDL tissue have been performed [8, 9, 10]; however, cryopreservation of teeth with the use of CAS freezers has never been investigated. The revascularization capability of transplanted teeth with incomplete root formation (immature teeth), which is related to pulp viability, is of the utmost importance. Although a few studies have been conducted to examine pulp reactions following cryopreservation [11, 12], consistent conclusions could not be achieved and some indistinct points still remain. According to previous statements, this clinical study was performed with the main purpose of examining the effects of cryopreservation on human periodontal ligament cells using a magnetic field freezer and evaluating the clinical influences of cryopreservation on pulp tissues of immature and mature human teeth.
Patient and Methods
Case report
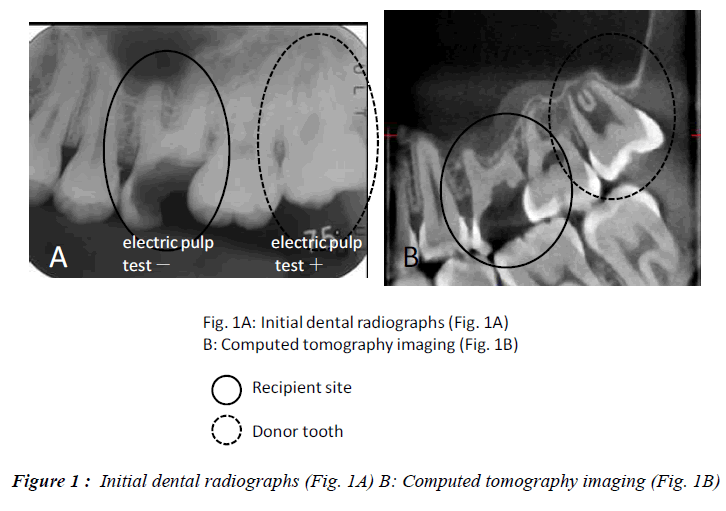
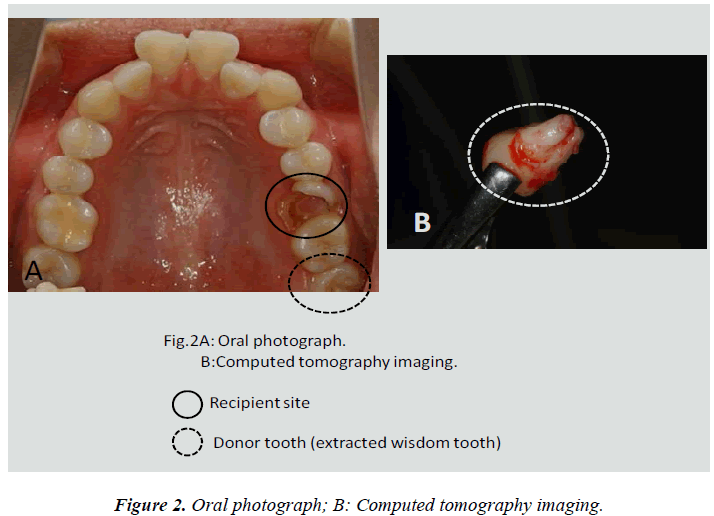
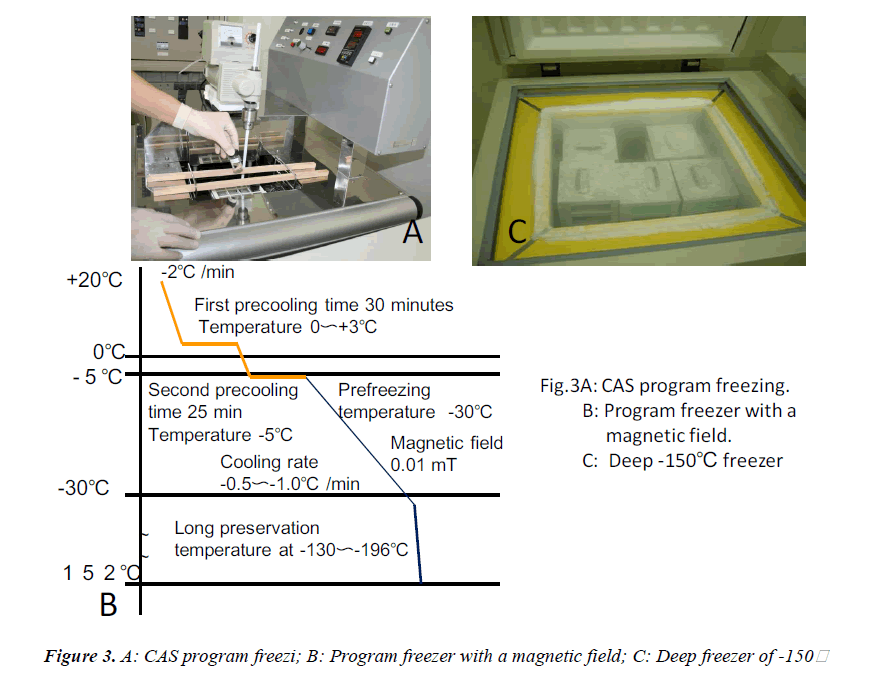
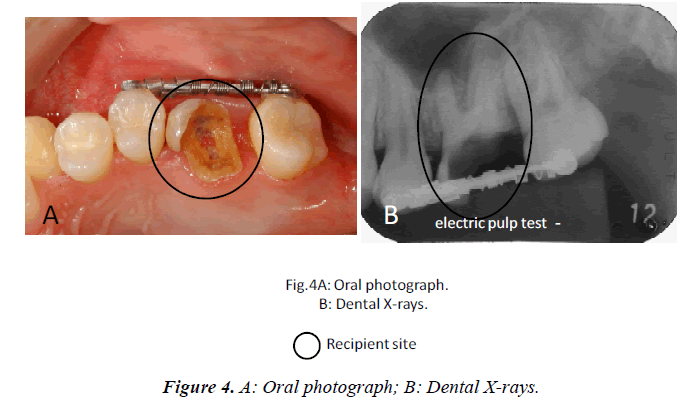
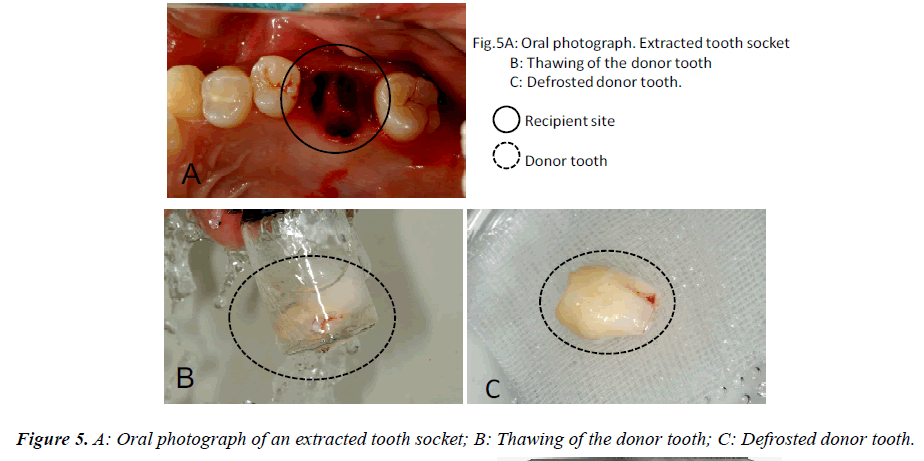
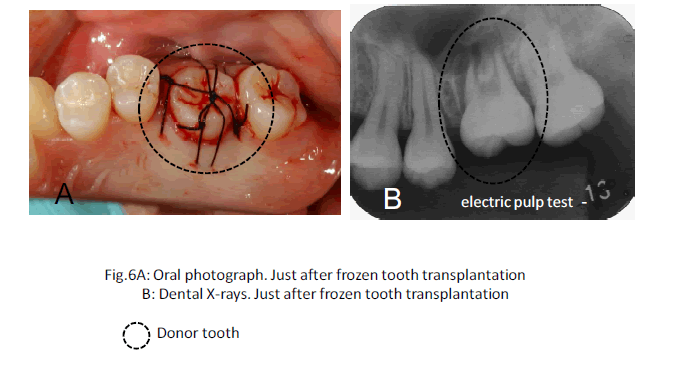
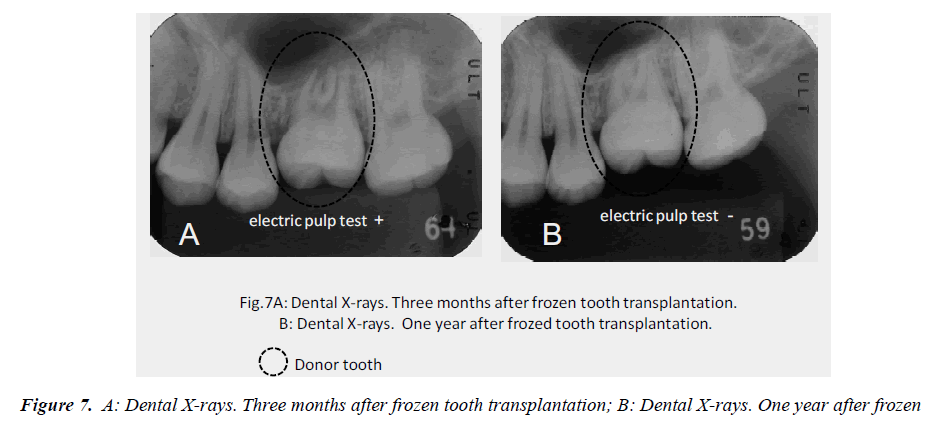
In this clinical study, we report a 20-year-old patient who came to our dental clinic. After initial dental radiographs (Fig. 1A) and computed tomography imaging (Fig. 1B) had been taken, caries as a result of second molar mesial inclination malposition was observed in the maxilla right first molar. It was decided to extract the first molar and transplant the third molar to the first molar area (Fig. 2A). This case had to be performed with distal movement of the second molar (Fig.1A and B). For this reason, the wisdom tooth was extracted (Fig. 2B), frozen in the CAS freezer, and cryopreserved (Fig. 3A and B) in the deep freezer for 3 months (Fig. 3C) until proper bone regenera tion had occurred, enabling transplantation. One month after donor tooth extraction, orthodontic brackets were bonded correct the tipped back second molar (Fig. 4 A and B). The hole of the reception side was prepared just before donor tooth transplantation by tooth extraction of the first molar (Fig. 5A). On the day of oral surgery, the cryopreserved tooth was thawed in tap water in a bottle (Fig. 5B) and washed several times in sterile saline (Fig. 5C). Transplantation was performed under local anesthesia by a maxillofacial surgeon (Fig. 6A and B). The transplanted tooth was placed slightly below the occlusal plane and splinted with sutures (Fig. 6A). Dental radiograph was taken right after the transplantation for recording and confirming position of the tooth (Fig. 6B). Sutures were removed 7 days postoperatively. The patient was called for a follow-up on the first month after transplantation and every 3-6 months after that for one year. On each visit, primary tests such as mobility and precaution, pocket depth, and tooth vitality with electrical pulp testing were examined and periapical or dental X-rays were taken (Fig. 7A and B).
Results
After the tooth was thawed and washed in sterile saline before transplantation, it was carefully monitored. No signs of tooth cracks or PDL and Hertwig’s tissue destruction were observed, thus the tooth was ready for transplantation (Fig. 5C). Radiography immediately taken after transplantation showed wide radiolucency at the PDL (Fig. 6B). Two weeks after transplantation, pain decreased and no sign or symptoms of inflammation could be observed, while the tooth remained unresponsive to electrical pulp testing and mobility was still grade 2. One to three months after transplantation, not only was no pathological radiolucency or tooth resorption noted, but also the disappearing radiolucency illustrated good PDL regeneration (Fig. 7A). Furthermore, the tooth stabilized and started to response to the electrical pulp test and some signs of closure of the root apex were visible radiographically. One year later, development of intact lamina dura around the tooth root and the trabecular bone around lamina dura indicated sufficient bone regeneration (Fig. 7B).. Although root length was still shorter than average, apex closure was completely obtained and complete apexogenesis could be achieved (Fig. 7B). Furthermore, the tooth stabilized and showed no reaction to the electrical pulp test and some signs of closure of the root apex and dental pulp were visible radiographically (Fig. 7B).
Discussion
After the first trial of third molar transplantation was performed by Apfel [13] and Miller [14] in the 1950s, several molar transplantations were reported by Nordenram [15], and Walker & Shaeffer [16]. Later, the concept of cryopreservation for storage of human teeth before transplantation, where immediate replantation was not possible or indicated, was created and followed by several investigations [17, 20]. After the development of an innovative programmed freezer with a magnetic field, an influential evolution occurred in the field of tooth banking [7, 21]. In our previous study [7], the importance of applying a magnetic field to cryopreservation of cells was confirmed. The survival rate of cells cryopreserved in the CAS freezer group was significantly higher than that of a normal freezer without a magnetic field. Although a high survival rate of PDL cells after short-term cryopreservation with a CAS freezer was confirmed, whether the pulp of the incomplete root of short-period cryopreserved periodontal ligament cells was included which is the key to success in tooth autotransplantation, was an issue that remained unanswered. In this study, PDL and pulp tissue cryopreserved for three months in the CAS freezer showed no differences in immediate autotransplantation. We performed CAS frozen teeth transplantation in humans [22- 24]. Immediately after transplantation, no response to thermal or electrical stimulation could be found in the cryopreserved immature tooth. This delay (three months after transplantation) was also perceived after transplantation of non-cryopreserved immature teeth [25]. However, transplanted teeth, regardless of being cryopreserved or not, will subsequently respond to electrical pulp testing. The probable reason is that regeneration of nerve fibers after transplantation, in general, takes a longer time than revascularization [26, 27]. Although reinnervation of damaged nerves in cryopreserved teeth with immature root formation needs a longer time than freshly extracted and transplanted teeth with immature root formation, it should be considered that nerve regeneration will only be deferred not eliminated. In addition, root formation after transplanting teeth also depends on the presence in the root apex of a healthy Hertwig’s epithelial root sheath (HERS). HERS plays an important role in root development and if damaged, normal root development will be interrupted [28]. Hence, further investigations regarding the characteristics of HERS cells after magnetic cryopreservation should be performed.
References
- Amos MJ. Day P, Littlewood SJ, Autotransplantation of teeth: an overview, Dent. Update 2009; 36: 102-104, 107-110, 113.
- Thomas S, Turner SR, Sandy JR. Autotransplantation of teeth: is there a role?, Br J Orthod. 1998; 25: 275-282.
- Takeda F. The effect of a magnetic field on water treatment, Water purification and liquid wastes treatment. 1991; 32: 515-517.
- Line SE, Polson AM, Zander HA. Relationship between periodontal injury, selective cell repopulation and ankylosis, J Periodontol 1974; 45: 725-730.
- Lindskog S, Blomlof L, Hammarstrom L. Repair of periodontal tissues in vivo and in vitro. J. Clin. Periodontol 1983; 10: 188-205.
- Melcher AH. Repair of wounds in the periodontium of the rat. Influence of periodontal ligament on osteogenesis. Arch. Oral Biol. 1970; 15: 1183-1204.
- Kaku M, Kamada H, Kawata T, Koseki H, Abedini S, Kojima S, Motokawa M, Fujita T, Ohtani J, Tsuka N, Matsuda Y, Sunagawa H, Hernandes RAM, Ohwada N, Tanne K. Cryopreservation of periodontal ligament cells with magnetic field for teeth banking, Cryobiology 2010; 61: 73-78.
- Izumi N, Yoshizawa M, Ono Y, Kobayashi T, Hamamoto Y, Saito C. Periodontal regeneration of transplanted rat teeth subcutaneously after cryopreservation, Int. J. Oral Maxillofac. Surg 2007; 36: 838-844.
- Oh YH, Che ZM, Hong JC, Lee EJ, Lee SJ, Kim J. Cryopreservation of human teeth for future organization of a tooth bank - a preliminary study, Cryobiology 2005; 51: 322-329
- Temmerman L, Dermaut LR, De Mil M, Van Maele G, Beele H, De Pauw GAM. Influence of cryopreservation on human periodontal ligament cells in vitro. Cell Tissue Bank. 2008; 9: 11-18.
- Price PJ, Cserepfalvi M. Pulp viability and the homotransplantations of frozen teeth. J Dent Res 1972; 51: 39-43.
- Schwartz O, Andreasen J.O, Greve T. Cryopreservation before replantation of mature teeth in monkeys. (II). Effect of preincubation, different freezing and equilibration rates and endodontic treatment upon periodontal healing. Int J Oral Surg 1985; 14: 350-361.
- Apfel H. Autoplasty of enucleated prefunctional third molars. J Oral Surg. 1950; 8: 289-296.
- Miller HM. Transplantation of teeth. NY State Dent J. 1951; 17: 382-386.
- Nordenram A. Autotransplantation of teeth. Acta Odontol Scand 1963; 21: 7-76.
- Walker RV, Schaffer R. Transplantation of teeth. (Series of 50 tooth transplantation) Baylor. Dent J. 1964; 14: 4-9.
- Bartlett PF, Reade PC. Cryopreservation of developing teeth, Cryobiology. 1972; 9: 205-211.
- Ohman A. Healing and sensitivity to pain in young replanted human teeth. An experimental, clinical and histological study, Odontol Tidskr. 1965; 73: 166-227.
- Schwartz O, Andreasen JO. Cryopreservation of mature teeth before replantation in monkeys (I). Effect of different cryoprotective agents and freezing devices. Int J Oral Surg 1983; 12: 425- 436.
- Schwartz O. Cryopreservation as long-term storage of teeth for transplantation or replantation. Int J Oral Maxillofac Surg 1986; 15: 30-32.
- Lee SY, Chiang PC, Tsai YH, Tsai SY, Jeng JH, Kawata T, Huang HM. Effects of cryopreservation of intact teeth on the isolated dental pulp stem cells. J Endo 2010; 36: 1336-1340.
- Kamada H, Kaku M, Kawata T, Koseki H, Abedini S, Kojima S, Sumi A, Motokawa M, Fujita T, Ohtani J, Ohwada N, Tanne K. In-vitro and in-vivo study of periodontal ligament cryopreserved with a magnetic field. Am J Orthod Dentofacial Orthop 2011; 140: 799-805.
- Kawata T, Kaku M, Motokawa M, Ueda H, Tanne K. The Teeth Bank and tooth transplantation ―Consideration from the History of Dental Treatment―Hiiroshima Univ Dent J 2005; 37: 137-141. In Japanese
- Kawata T, Kohno S, Kaku M, Fujita T, Ohtani J, Motokawa M, Tanne K. Expression of vascular endothelial growth factor on neovascularization during experimental tooth movement by magnets. Biomed Res, 2011; 22: 248-253.
- Keightley AJ. Cross DL. McKerlie RA. Brocklebank L. Autotransplantation of an immature premolar, with the aid of cone beam CT and computer-aided prototyping: a case report. Dent Traumatol 2010; 26: 195-199.
- Ohman A. Healing and sensitivity to pain in young replanted human teeth. An experimental, clinical and histological study, Odontol Tidskr. 1965; 73: 166-227.
- Schendel KU, Shwartz O, Andreasen JO, Hoffmeister B. Reinnervation of autotransplanted teeth. A histological investigation in monkeys, Int J Oral Maxillofac Surg. 1990; 19: 247-249.
- Friedlander LT, Cullinan MP, Love RM. Dental stem cells and their potential role in apexogenesis and apexification, Int Endod J. 2009; 42: 955-962.