Research Article - Biomedical Research (2017) Volume 28, Issue 11
Effects of 1, 25-dihydroxyvitamin D3 on expression of TGF-β1, CD68 and MCP-1 in type 2 diabetic nephropathy rat
Xiaoyun Zeng1,2#, Fapeng Li3#, Li Quan1,2, Hua Yao2,3 and Jun Zhu1,2,4*
1Department of Endocrinology, the First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, PR China
2Diabetes Prevention, Diagnosis and Treatment Center, the First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, PR China
3Cardiac Intensive Care Unit, the First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, PR China
4Xinjiang Key Laboratory of Metabolic Disease, the First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, PR China
#These authors contributed equally to this work
- *Corresponding Author:
- Jun Zhu
Department of Endocrinology
The First Affiliated Hospital of Xinjiang Medical University, PR China
Accepted on March 22, 2017
Abstract
Objective: This study aims to investigate the role and mechanism of 1, 25-dihydroxyvitamin D3 in type 2 diabetic nephropathy rats.
Methods: There were 30 male Sprague-Dawley (SD) rats that were randomly divided into 3 groups: the normal control group (NC group, n=10), the diabetic nephropathy model group (T2DN model group, n=10), and the diabetic nephropathy group treated with 1, 25-dihydroxyvitamin D3 (vD3-T2DN group, n=10). After 15 weeks, the changes of renal tissues morphology, renal function, and 24 h urinary protein quantification were measured. The expressions of Transforming Growth Factor beta 1 (TGF-β1), CD68 and Monocyte Chemoattractant Protein-1 (MCP-1) in renal cortex were also detected by Immunohistochemistry (IHC).
Results: The weight of rats were significantly decreased in T2DN model group and vD3-T2DN group than in NC group (P<0.05) at the end of 15th week, while the blood glucose, 24 h urinary protein and triglyceride were significantly increased (P<0.01). The expression of TGF-β1, CD68 and MCP-1 in T2DN model group and vD3-T2DN group were significantly higher than in NC group (P<0.01). Compared with NC group, the triglyceride (P<0.01) and serum creatinine (P<0.05) were significantly higher in T2DN model group. In vD3-T2DN group, the expression of TGF-β1, CD68 and MCP-1, the content of 24 h urinary protein (P<0.01), and triglyceride (P<0.05) were significantly than in T2DN model group.
Conclusion: Through repressing the expression of TGF-β1, CD68 and MCP-1, 1, 25-dihydroxyvitamin D3 can inhibit invasion of macrophages to protect kidney of T2DN rats.
Keywords
Type 2 diabetic nephropathy, 1, 25-dihydroxyvitamin D3, Transforming growth factor beta 1 (TGF-β1), CD68, Monocyte chemoattractant protein-1 (MCP-1)
Introduction
Diabetic Nephropathy (DN) is one of common and serious chronic complication of Diabetes Mellitus (DM). DN is a severe microvascular complication, which is the common etiology of end-stage renal diseases [1,2]. In recently, a large number of studies [3-6] found that immune inflammatory cells, cytokines, acute phase reactive protein and other inflammatory mediators played important roles in the genesis and development of DN [7]. It is found that Monocyte Chemoattractant Protein-1 (MCP-1) is the main factor for monocyte-macrophage cell aggregation, which directly stimulated mesangial cell to express Transforming Growth Factor beta 1 (TGF-β1) to accelerate renal fibrosis [8]. TGF-β1 is a powerful fibrosis factor, which is involved in glomerular hypertrophy and Extracellular Matrix (ECM) progressive accumulation process through promoting ECM components accumulation, such as synthesis of collagen and fibronectin proteins and inhibition of degradation enzymes for ECM components, and ultimately leads to glomerulosclerosis [9-11].
Recent studies showed that 1, 25-dihydroxyvitamin D3 (1, 25- (OH)2 D3, active vitamin D3, calcitriol) is not only involved in the incidence of type 2 diabetes, but also protects kidney of DN by reducing urinary protein, inhibiting aggregation of ECM, epithelial-mesenchymal, anti-inflammation and Renin Angiotensin Aldosterone System (RAS) [12-18]. In this study, we investigated the protective roles of vitamin D3 in type 2 DN (T2DN) rats and explored the related molecular mechanisms.
Materials and Methods
Animal modeling and grouping
Totally 30 male SD rats (provided by Experimental Animal Center, Xinjiang Medical University) with weighing about 80 g, were divided into 3 groups. The Normal Control group (NC group) was fed with normal diet, while the diabetic nephropathy model group (T2DN model group) and the 1, 25- dihydroxyvitamin D3 treatment diabetic nephropathy group (vD3-T2DN group) were fed with high-fat and high-sugar diets (containing 10% refining lard, 20% sucrose, 2% cholesterol, 1% pig bile and 67% of normal diet). After 6 weeks, the T2DN model group and vD3-T2DN group were subjected to the tail intravenous injection with 30 mg/kg STZ (Sigma, St. Louis, MO, USA), which dissolved in pH4.5 citrate buffer after 12 h fasting. One week later, the Fasting Plasma Glucose (FPG) and 2 h plasma glucose (2hPG) were tested, and rats with FPG ≥ 7.0 mmol/L and/or 2hPG ≥ 11.1 mmol/L were considered as Type 2 diabetic (T2DN) models.
The test strip for urinary microproteins was used to measure continuously the morning urine of the T2DN rats after STZ injection at six weeks and seven weeks. If the results were positive, these rats were labeled as T2DN model, and they were continuously measured 24 h proteinuria. The T2DN model rats were given by gavage with 0.03 μg/kg vitamin D3, dissolved in 0.05 ml peanut oil. Meanwhile, the T2DN model group were also treated by equal amount of peanut oil. Three groups of rats were sacrificed after 15 weeks. About 4 to 6 milliliters of venous blood and 24 h-urine prior to sacrifice were collected, and bilateral kidneys were removed and weighted. Renal cortex of 1 mm3 was obtained from the kidney, and then fixed in 2.5% glutaraldehyde. Remaining kidney tissues were fixed in 4% paraformaldehyde.
Detection of biochemical parameters
Enzyme immunosorbent assay kit (Beyotime Institute of Biotechology, Haimen, Jiangsu, China) was adopted to measure 24 h-urinary albumin. Hitachi 7600 Automatic Biochemical Analyzer (Hitachi Science and Systems, Tokyo, Japan) was used to detect the content of blood glucose, creatinine, urea nitrogen, cholesterol and triglycerides.
Light microscopy examination
The light microscope samples were fixed in 4% paraformaldehyde and dehydrated by different concentration of alcohol (70%, 80%, 90%, 95%, and 100%). After hyalinized by xylene, these samples were embedded with paraffin, and then continuously cut into 2 μm sections. Hematoxin Eosin (HE) staining, Periodic Acid Schiff (PAS) reaction, Periodic-Acid-Silver Metheramine (PASM) staining and Masson’s trichrom staining were performed before light microscope examination.
Transmission electron microscopy examination
The samples for transmission electron microscope oil were eluted with alcohol. After cutting into 1 mm3 sections, the tissue was immediately fixed with glutaraldehyde liquid and osmic acid at 4°C. After dehydrated by acetone and alcohol, the samples were moved into acetone with epoxy resin, then cut into ultramicrocuts on ultramicrotome with 70-90 nm thickness after epoxy resin embedding, drying, fast repair and location. The sections were stained with uranyl acetate and lead Azusa citric acid, and then subjected to transmission electron microscopy on JEM100CX 2II electron microscope (Image Processing Center of Beihang University, Beijing, China).
Immunohistochemistry (IHC)
Paraffin sections were treated with normal procedures, then incubated with Rabbit anti-rat MCP-1 polyclonal antibody (l: 100 dilution; Boster Biological Technology, Wuhan, Hubei, China), mouse anti-rat CD68 (l: 200 dilution; Boster Biological Technology, Wuhan, Hubei, China) and TGF-β1 polyclonal antibody (l: 100 dilution; Santa Cruz, Texas, USA), at 37°C for 30 min respectively. The negative control was also dealt with PBS rather than antibody under same condition. Then secondary antibodies were added to incubate the sections. After stained with DAB chromogenic reagent (Beijing ZhongshanJinqiao Biotechnology Co., Ltd, Beijing, China) and counterstained with hematoxylin, these sections were visualized under microscopy with the CM-2000B biomedicine image analysis system (Beihang, Beijing, China). Five fields were randomly selected under high magnification (X400), and brown staining was considered as positive. The average number of positive cells in every 2 mm2 were counted and calculated.
Statistical analysis
SPSS 17.0 software was used for statistical analysis, and data were expressed as mean ± Standard Deviation (SD). ANOVA was used for the group comparison and pairwise comparison. Chi-square test was used to analyse enumeration data. Correlation analysis was used to analyse the relationship of two variables, and P<0.05 was considered statistically significant.
Results
Comparison of biochemical characters among NC group, T2DN model group and vD3-T2DN group
In order to investigate the effect of vitamin D3 on biological characters in T2DN model rats, we firstly measured the body weight, kidney weight, blood glucose content, 24 h urinary protein, triglycerides, cholesterol, blood urea nitrogen and creatinine content among NC group, T2DN model group and vD3-T2DN group. As shown in Table 1, the body weight and kidney weight was significantly lower in T2DN model group and vD3-T2DN group than in NC group (P<0.05), while the blood glucose and 24 h urinary protein was significant higher in T2DN model group and vD3-T2DN group than in NC group (P<0.05). However, the 24 h urinary protein was significant lower in vD3-T2DN group when compared with T2DN model group. We also found that the concentration of triglycerides, cholesterol and creatinine in T2DN model group were all significant higher than in NC group. It was shown that the content of triglycerides was significant decreased in vD3- T2DN groups compared with T2DN model group (Table 2).
| Group | Number of rats | Body weight (g) | Renal weight (g) | Blood glucose (mmol/L) | Proteinuria (g/24 h) |
|---|---|---|---|---|---|
| NC group | 10 | 486.8 ± 33.29 | 4.00 ± 0.34 | 6.96 ± 9.76 | 0.06 ± 0.51 |
| T2DN group | 10 | 404.8 ± 63.52* | 3.92 ± 0.62 | 27.40 ± 2.96** | 1.15 ± 0.34** |
| vD3-T2DN group | 10 | 414.6 ± 52.83* | 3.98 ± 0.47 | 25.95 ± 4.62** | 0.33 ± 0.14**▲▲ |
| Note: Compared with NC group, *P<0.05, **P<0.01; compared with T2DN group, ▲P<0.05, ▲▲P<0.01. | |||||
Table 1. Comparison of body weight, renal weight, blood glucose and 24 h urinary protein excretion among different groups (mean ± SD).
| Group | Number of rats | Triglycerides (mmol/L) | Cholesterol (mmol/L) | Blood urea nitrogen (mmol/L) | Creatinine (mmol/L) |
|---|---|---|---|---|---|
| NC group | 10 | 0.88 ± 0.92 | 0.78 ± 0.38 | 8.89 ± 0.80 | 44.2 ± 4.64 |
| T2DN group | 10 | 8.30 ± 5.15** | 28.10 ± 19.47** | 11.78 ± 4.62 | 306.40 ± 257.94* |
| vD3-T2DN group | 10 | 4.28 ± 3.94▲ | 28.79 ± 24.26** | 10.95 ± 3.33 | 180.11 ± 329.33 |
| Note: Compared with NC group, *P<0.05, **P<0.01; compared with T2DN group, ▲P<0.05, ▲▲P<0.01. | |||||
Table 2. Comparison of the content of triglycerides, cholesterol, blood urea nitrogen and creatinine among different groups (mean ± SD).
Light microscopy examination of the renal tissues among NC group, T2DN model group and vD3-T2DN group
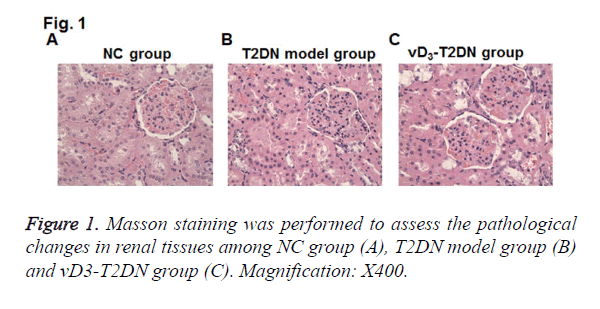
To elucidate the effects of vitamin D3 on renal pathology in T2DN model rats, Masson staining on renal tissues was performed. Compared with NC group, mesangial cells were hyperplasia from mild to moderate in T2DN model group, and the area of mesangial area was increased in the glomerular capillary (Figure 1). The tubulars were expanded and infiltrated with a large number of inflammatory cells. Compared to T2DN model group, kidney tubulars were slightly expanded and less inflammatory cells were infiltrated in vD3-T2DN group than in T2DN model group.
TEM examination of the renal tissues among different groups
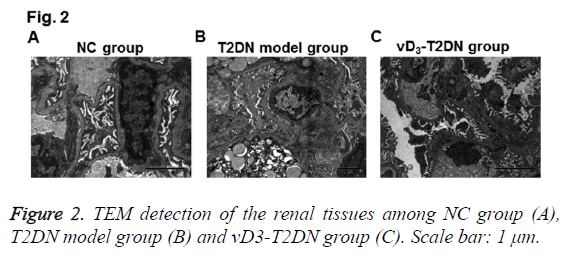
To further confirm the therapeutic effects of vitamin D3 on renal tissues in T2DN, TEM detection was also performed. As shown in Figure 2, several characters were observed in T2DN model group rather than in NC group, such as mesangial endothelium swelling, lipid droplets hyperplasia, podocytes Golgi proliferation and actin enrichment, endotheliocytes vacuole degeneration, renal tubulars nuclear condensation, nuclear gap widened, thick clumps of heterochromatin and endoplasmic reticulum expansion. While in the vD3-T2DN group, mild edema and denser massive structures were obvious in podocytes, valuolar degeneration changed slightly in endotheliocyte and mesangial cells, capsular space and vessel opened less, fat degenerated slighter in lipid droplets, and podocytes edema was slighter. These results validated the therapeutic effects of vitamin D3 in T2DN.
Expression of CD68, MCP-1 and TGF-β1 among NC group, T2DN model group and vD3-T2DN group
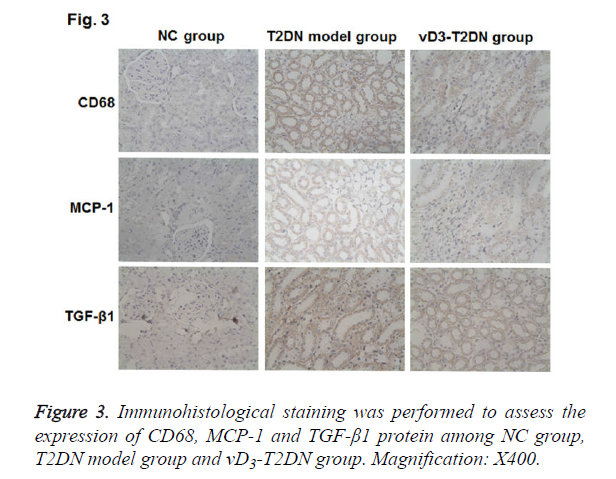
To investigate the molecular mechanism of vitamin D3 in the therapy on the T2DN, IHC was performed to detect the expressions of CD68, MCP-1 and TGF-β1 in renal tissues. As shown in Figure 3 and Table 3, CD68, MCP-1 and TGF-β1 were mainly expressed in the renal tubules and interstitial cytoplasm. The expression of CD68, MCP-1 and TGF-β1 were hardly detected in NC group, while they were highly expressed in T2DN model group and vD3-T2DN group (P<0.01). In the vD3-T2DN group, the expression of CD68, MCP-1 and TGF- β1 were higher than in NC group, and they were lower than in T2DN model group. These results indicated that vitamin D3 treatment could regulate the expression of CD68, MCP-1 and TGF-β1, which contribute to its protection effect in T2DN.
| Group | TGF-β1 | MCP-1 | CD68 |
|---|---|---|---|
| NC group | 2.58 ± 0.68 | 2.20 ± 0.79 | 2.56 ± 1.06 |
| T2DN group | 16.2 ± 2.12**▲▲ | 15.3 ± 2.70**▲▲ | 7.72 ± 2.04**▲▲ |
| vD3-T2DN group | 5.02 ± 1.75**▲▲ | 7.66 ± 3.09**▲▲ | 7.72 ± 2.04**▲▲ |
| Note: Compared with NC group, *P<0.05, **P<0.01; compared with T2DN group, ▲P<0.05, ▲▲P<0.01. | |||
Table 3. Comparison of TGF-β1, MCP-1 and CD68 expression among different groups (mean ± SD).
Discussion
In recent years, several studies revealed that the key factors for the development of DN were inflammation and macrophages infiltration [19]. Moreover, the infiltration of mononuclear macrophages in renal tissues are correlated with accumulation of glomerular Extracellular Matrix (ECM) in glomerular mesangial and renal tubular, which is the characteristic pathological changes of DN [20]. MCP-1 is a cytokine secreted by renal tubular epithelial and mesangial cells, and its main function is to activate mononuclear macrophage, and its expression in renal tissue can serve as a biomarker of inflammation [8,21-23]. Cheng et al. found that MCP-1 could combine with glomerular mesangial cell membrane protein CCR2, which led to activate NK-κB pathway to induce TGF- β1 expression and increase the expression of fibronectin (Fn) in mRNA level and protein level. It was verified that the active TGF-β1 in DN was related to deposition of glomerular ECM [9], expression of type I collagen in mesangial cells [24], increased expression of Fn [25] protein and worsen renal function of DN.
Studies have shown that vitamin D3 plays its biological roles in cells through combining to vitamin specific receptor (VDR) [26-28]. Li et al. found that the diabetic mice with VDR deletion showed strongly active Renin-Angiotensin System (RAS) during their growth and development stage, accompanied with severe renal impairment [29]. However, the kidney injury was significantly alleviated through inhibition of RAS and therapy with vitamin D analogues. Zhang et al. confirmed that vitamin D3 could inhibit the expression of renin and TGF-β1, reduce proteinuria and delay progression of renal failure through giving 1, 25-(OH)2 D3 to VDR knockout mouse model [30].
In this study, we firstly established T2DN rat model, and found that proteinuria and TGF -β1, MCP-1 and CD68 expression was significant higher in T2DN model group than in the NC group, while it was lower than in the vD3-T2DN group. These results indicated that active vitamin D3 could repress the expression of TGF-β1, MCP-1 and CD68, and protect the integrity of glomerular filtration membrane and diminish proteinuria. We also found that the body weight decreased significantly in T2DN model group and vD3-T2DN group than in NC group. At the end of week 15, the content of glucose, 24 h urine protein and cholesterol was significantly increased in T2DN model group and vD3-T2DN group than in NC group, as well as the expression of CD68, MCP-1, TGF-β1. Moreover, the content of triglycerides and creatinine was also significantly up-regulated in T2DN model group than in NC group. For the expression of CD68, MCP-1 and TGF-β1, it was similar to Tian et al. [31] when compared vD3-T2DN group with T2DN model group. Meanwhile, the 24 h urine protein and triglycerides were significantly decreased in vD3-T2DN group.
Furthermore, our results showed that the 24 h urine protein was obviously decreased in vD3-T2DN group, whose kidney injuries were also alleviated, compared with T2DN model group. This result indicated that 1, 25-(OH)2 D3 could protect kidney in T2DN rats. In this study, it was indicated that Vitamin D may reduce proteinuria in diabetic nephropathy, which was consistent with reported clinical trials [32].
We also investigated the protection mechanism of 1, 25-(OH)2 D3 effect on T2DN rats and found that the infiltration of macrophages were significantly decreased in kidney tissues of vD3-T2DN rats, and 1, 25 (OH)2 D3 could reduce the expression of CD68, MCP-1 and TGF-β1 [31]. TGF-β can be induced by angiotensin II, which is a powerful factor for mediating fibrosis [33]. Through stimulating the synthesis of extracellular matrix proteins and reducing the degradation of matrix proteins, TGF-β can promote apoptosis of foot cells, which plays a key role in diabetic glomerulosclerosis [34]. And MCP-1 is monocyte chemoattractant protein-1, which can lead to kidney tissue injury through activating inflammatory cells and prompting the release of soluble medium from inflammatory cells [35]. CD68 is one kind of macrophage marker, and macrophage infiltration plays as an important mediator for renal interstitial fibrosis [36]. In this study, it was indicated that the expressions of TGF-β1, MCP-1 and CD68 were decreased in kidney tissues of DN rat, which further validated that vD3 can alleviate diabetic nephropathy through inhibiting macrophage infiltration in kidney and relieving inflammatory pathways.
Vitamin D could not affect the blood glucose content, because the content of blood glucose showed no difference between in T2DN model group and in vD3-T2DN group. This result indicated that the renal protective effect of vitamin D may be not reached by lowering glucose and reducing glycosylated productions. Zhao et al. developed a clinical trial that also indicated that vitamin D can protect diabetic nephropathy independent of the control of blood glucose and blood pressure [37].
Our study suggests that 1, 25-(OH)2 D3 could play protective roles in kidney through reducing the expression of MCP-1 in renal tissues, which inhibits the infiltration of macrophages and down-regulates TGF-β1 expression.
Acknowledgements
This work was supported by National Natural Science Foundation of China grant (No. 81160116).
Disclosures
All authors declare no financial competing interests. All authors declare no non-financial competing interests.
References
- Pugliese G. Updating the natural history of diabetic nephropathy. Acta Diabetol 2014; 51: 905-915.
- Ahmad J. Management of diabetic nephropathy: Recent progress and future perspective. Diabetes Metab Syndr 2015; 9: 343-358.
- Perlman AS, Chevalier JM, Wilkinson P, Liu H, Parker T, Levine DM, Sloan BJ, Gong A, Sherman R, Farrell FX. Serum Inflammatory and immune mediators are elevated in early stage diabetic nephropathy. Ann Clin Lab Sci 2015; 45: 256-263.
- Zhang C, Xiao C, Wang P, Xu W, Zhang A. The alteration of Th1/Th2/Th17/Treg paradigm in patients with type 2 diabetes mellitus: Relationship with diabetic nephropathy. Hum Immunol 2014; 75: 289-296.
- Zhang Y, Ma KL, Liu J, Wu Y, Hu ZB, Liu L, Lu J, Zhang XL, Liu BC. Inflammatory stress exacerbates lipid accumulation and podocyte injuries in diabetic nephropathy. Acta Diabetologica 2015; 1-12.
- Donate-Correa J, Martín-Nunez E, Muros-de-Fuentes M, Mora-Fernandez C, Navarro-Gonzalez JF. Inflammatory cytokines in diabetic nephropathy. J Diabetes Res 2015; 2015: 948417.
- Mora C, Navarro JF. The role of inflammation as a pathogenic factor in the development of renal disease in diabetes. Curr Diab Rep 2005; 5: 399-401.
- Shaker O, Sadik N. Transforming growth factor beta 1 and monocyte chemoattractant protein-1 as prognostic markers of diabetic nephropathy. Hum Exp Toxicol 2013; 32: 1089-1096.
- Koga K, Yokoi H, Mori K, Kasahara M, Kuwabara T, Imamaki H, Ishii A, Mori KP, Kato Y, Ohno S, Toda N, Saleem MA, Sugawara A, Nakao K, Yanagita M, Mukoyama M. MicroRNA-26a inhibits TGF-ß-induced extracellular matrix protein expression in podocytes by targeting CTGF and is downregulated in diabetic nephropathy. Diabetologia 2015; 58: 2169-2180.
- Zhang Q, Lu Y, Ma Z, Li Y, Guo J. A novel formula from mulberry leaf ameliorates diabetic nephropathy in rats via inhibiting the TGF-β1 pathway. Food Funct 2015; 6: 3307-3315.
- Guo X, Zhou G, Guo M, Cheung AK, Huang Y, Beddhu S. Adiponectin retards the progression of diabetic nephropathy in db/db mice by counteracting angiotensin II. Physiol Rep 2014; 2: 00230.
- Sánchez-Hernandez RM, Garcia-Canton C, Lorenzo DL, Quevedo V, Bosch E, Lopez-Rios L, Riano M, Boronat M. The specific relationship between vitamin D deficiency and diabetic nephropathy among patients with advanced chronic kidney disease: a cross-sectional study in Gran Canaria, Spain. Clin Nephrol 2015; 83: 218-224.
- Chokhandre MK, Mahmoud MI, Hakami T, Jafer M, Inamdar AS. Vitamin D and its analogues in type 2 diabetic nephropathy: a systematic review. J Diabetes Metab Disord 2015; 14: 58.
- Derakhshanian H, Shab-Bidar S, Speakman JR, Nadimi H, Djafarian K. Vitamin D and diabetic nephropathy: A systematic review and meta-analysis. Nutrition 2015; 31: 1189-1194.
- Zhang XL, Guo YF, Song ZX, Zhou M. Vitamin D prevents podocyte injury via regulation of macrophage M1/M2 phenotype in diabetic nephropathy rats. Endocrinology 2014; 155: 4939-4950.
- Guan X, Yang H, Zhang W, Wang H, Liao L. Vitamin D receptor and its protective role in diabetic nephropathy. Chin Med J (Engl) 2014; 127: 365-369.
- Shoukry A, Bdeer SE-A, El-Sokkary RH. Urinary monocyte chemoattractant protein-1 and vitamin D-binding protein as biomarkers for early detection of diabetic nephropathy in type 2 diabetes mellitus. Mol Cell Biochem 2015; 408: 25-35.
- Fernandez-Juarez G, Luno J, Barrio V, de Vinuesa SG, Praga M, Goicoechea M, Lahera V, Casas L, Oliva J, PRONEDI Study Group. 25 (OH) vitamin D levels and renal disease progression in patients with type 2 diabetic nephropathy and blockade of the renin-angiotensin system. Clin J Am Soc Nephrol 2013; 8: 1870-1876.
- Shikata K, Makino H. Role of macrophages in the pathogenesis of diabetic nephropathy. Contrib Nephrol 2001; 46-54.
- Usui HK, Shikata K, Sasaki M, Okada S, Matsuda M, Shikata Y, Ogawa D, Kido Y, Nagase R, Yozai K, Ohga S, Tone A, Wada J, Takeya M, Horiuchi S, Kodama T, Makino H. Macrophage scavenger receptor-a-deficient mice are resistant against diabetic nephropathy through amelioration of microinflammation. Diabetes 2007; 56: 363-372.
- Fufaa GD, Weil EJ, Nelson RG, Hanson RL, Knowler WC, Rovin BH,Wu H, Klein JB, Mifflin TE, Feldman HI, Vasan RS, Kimmel PL, Kusek JW, Mauer M, CKD Biomarkers Consortium and the RASS Investigators. Urinary monocyte chemoattractant protein-1 and hepcidin and early diabetic nephropathy lesions in type 1 diabetes mellitus. Nephrol Dial Transplant 2015; 30: 599-606.
- Raina P, Matharoo K, Bhanwer A. Monocyte Chemoattractant Protein-1 (MCP-1) g.-2518 A>G polymorphism and susceptibility to Type 2 Diabetes (T2D) and End Stage Renal Disease (ESRD) in the North-West Indian population of Punjab. Ann Hum Biol 2014; 1-7.
- Panee J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine 2012; 60: 1-12.
- Castro NE, Kato M, Park JT, Natarajan R. Transforming Growth Factor beta1 (TGF-beta1) enhances expression of profibrotic genes through a novel signaling cascade and microRNAs in renal mesangial cells. J Biol Chem 2014; 289: 29001-29013.
- Alvarez ML, Khosroheidari M, Eddy E, Kiefer J. Role of microRNA 1207-5P and its host gene, the long non-coding RNA Pvt1, as mediators of extracellular matrix accumulation in the kidney: implications for diabetic nephropathy. Plos One 2013.
- Cheskis B, Freedman LP. Ligand modulates the conversion of DNA-bound vitamin D3 receptor (VDR) homodimers into VDR-retinoid X receptor heterodimers. Mol Cell Biol 1994; 14: 3329-3338.
- Li JJ, Kim RH, Zhang Q, Ogata Y, Sodek J. Characteristics of vitamin D3 receptor (VDR) binding to the vitamin D response element (VDRE) in rat bone sialoprotein gene promoter. Eur J Oral Sci 1998; 106: 408-417.
- Alonso C, Diaz N, Diaz-Corte C, Martin J, Cannata Andia J. Vitamin D receptor gene(VDR) polymorphisms: effect on bone mass, bone loss and parathyroid hormone regulation. Nephrol Dial Transplant 1998; 13: 73-77.
- Li YC. Vitamin D and diabetic nephropathy. Curr Diab Rep 2008; 8: 464-469.
- Zhang Z, Sun L, Wang Y, Ning G, Minto AW. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int 2008; 73: 163-171.
- Tian Y, Lv G, Yang Y, Zhang Y, Yu R, Zhu J, Xiao L, Zhu J. Effects of vitamin D on renal fibrosis in diabetic nephropathy model rats. Int J Clin Exp Pathol 2014; 7: 3028-3037.
- Joergensen C, Tarnow L, Goetze J, Rossing P. Vitamin D analogue therapy, cardiovascular risk and kidney function in people with type 1 diabetes mellitus and diabetic nephropathy: A randomized trial. Diabetic Med 2015; 32: 374-381.
- El Mesallamy HO, Ahmed HH, Bassyouni AA, Ahmed AS. Clinical significance of inflammatory and fibrogenic cytokines in diabetic nephropathy. Clin Biochem 2012; 45: 646-650.
- Bondar I, Klimontov V, Parfenteva E, Romanov V, Nadeev A. Urinary excretion of fibrogenic and antifibrotic growth factors in type 1 diabetic patients: the interrelationship with diabetic nephropathy. Terapevt Arkh 2011; 84: 36-40.
- Feng M, Xu C, Wen J, Lin G, Lv Q, Huang G. Effect of advanced glycosylation end products on oxidative stress and MCP-1 in human renal mesangial cells. Chin J Appl Physiol 2014; 30: 306-310, 313.
- Lewis A, Steadman R, Manley P, Craig K, de la Motte C, Hascall V, Phillips AO. Diabetic nephropathy inflammation, hyaluronan and interstitial fibrosis. Histol Histopathol 2008; 23: 731-739.
- Zhao J, Dong J, Wang H, Shang H, Zhang D. Efficacy and safety of vitamin D3 in patients with diabetic nephropathy: a meta-analysis of randomized controlled trials. Chin Med J (Engl) 2014; 127: 2837-2843.