- Biomedical Research (2015) Volume 26, Issue 1
Effect of ZnO NPs on tumor marker hormones in male rats.
Habibollah Nazem1 and Zahra Arefian2*1Department of Biochemistry, Isfahan University of Payame-Noor, Isfahan, Iran
2Department of Biochemistry, Taft University of Payame-Noor, Yazd, Iran
Accepted date: September 01 2014
Abstract
ZnO nanoparticles (NPs) are engineered nanoparticles used in many commercial products inclusive of cosmetics, paints, textiles, and sanitary products. ZnO NPs are potent in protecting the skin against UV radiation so, they are used as ingredients in sunscreen and moisturizing creams/lotions and hence the study of their toxicity is essential. On the one hand, nanoparticles, due to their small size occupy a significant place in cutting-edge applications in modern industry, while on the other hand; the small size of NPs may lead to their accumulation in the body leading to health hazards in human beings .The effect of ZnO NPs in different concentrations on Prostate Specific Antigen (PSA), 1- α- fetoprotein (AFP), and carcinoembryonic antigen (CEA) of male Wistar rats assigned in four groups, each consisting of 8 rat, was investigated. ZnO NPs was gavage-fed to groups 1, 2, and 3 with concentrations of 100, 200, and 400 mg/kg respectively for 10 days. A separate group of animals was assigned as the control group. Subsequent to the phase, tumor marker hormones AFP, CEA, and PSA concentrations were estimated. Results showed that ZnO NPs is toxic in the animal model. Thus, it is proposed that the NPs should be applied taking into consideration the health hazard that it might cause and a continuous monitoring of individuals’ health is essential.
Keywords
ZnO, tumor marker, AFP, CEA, PSA.
Introduction
Zinc is a rare and essential element in human body and ZnO is used as a zinc source in the nutrition industry. NPs have individual characteristics that make them appropriate for application in diagnosis, drug delivery, nutrition industry, paint, environment decontamination, cosmetics and sunscreens. Due to a wide use of nanotechnology in development, human exposure to NPs has been increased. ZnO NPs are used in many commercial products such as cosmetics, paints, textiles, and sanitary products. They also have high photo-stability and low photo-allergic potential, Titanium oxide (TiO2) and ZnO NPs provide a profound protection against UV radiations; and hence are used widely in sunscreen products also Due to their antimicrobial properties ZnO NPs are used in nutrients as additives. They have anti-fungal property which are operated in agriculture sectors and is also deployed in anticancer characteristics in medicines.
Experiments with intestinal cell culture showed that ZnO NPs in small sizes are more toxic compared to their larger versions, NPs typically lead to Inflammation and Fibrosis and damages the DNA. Exposure of epithelial cells to ZnO NPs leads to acute toxicity, oxidative stress and genomic toxicity. However, these effects are not so acute in in vitro experiments. There are reports; which show that inhalation of ZnO NPs cause cell toxicity and inflammation. Studies have also shown that after oral administration, kidneys and spleen are the target organs of NPs. Previous reports state that when a 50 mg/kg and a 300 mg/kg ZnO nanoparticle dose is exploited, elevated zinc levels were observed in the liver, lung, and kidney of rats even after 6–24 hours this rises from the ionization property of NPs after oral administration. However, there is little information on chronic or sub-chronic toxicity of ZnO NPs. In this study ZnO NPs were gavage fed in 3 doses for 10 days to determine the toxicity of NPs in the concentrations of tumor markers hormones with respect to Prostate Specific Antigen (PSA), 1- α- fetoprotein (AFP), and carcinoembryonic antigen (CEA) in male rats.
Materials and Methods
Equipment
1. Centrifuge (Eppendorf)
2. Electrochemical luminance device
3. Transmission electron microscope (JEM-200CX) and Broker X-ray diffraction device with radiation of Cu-Kα (TU-1901 double-beam).
4. Biochemical tests were accomplished by electrochemical luminance device Elecsys 2010 co-produced by HITACHI Japan and ROCHE Germany for all the experiments relevant to hormones
5. Tumor markers, Hepatitis panel, and AIDS by ultrasensitive electro-chemiluminescence fully automatic, employing specific kits.
Materials
Ketamine was used as anesthesia for the rodents. Laboratory kits of AFP, CEA, and PSA were provided by PARS AZMOON enterprise holding a Referenced laboratorial certificate. ZnO NPs were the required materials for this test in size 20-30 nm and doubled distilled water.
Animals
Male Wistar rats were used for this experiment. Thirtytwo rats aged 8 weeks and weighing 200-250 g were divided into 4 groups (one control and 3 experimental groups) each consisting of 8 Rats. The animals obtained from Pasteur Institute were kept in cages under conditions of temperature 22±10 °C, humidity %60±10, with12h light and dark cycle and access to water and food ad libidum. The rodents were housed in this condition for 2 weeks prior to the experiment, for adaptation. All experiments were conducted in accordance with the Ethical Committee Act. Treatment group rats were marked with stains and a suspension of ZnO NPs was gavage-fed to the rodents in doses of 100, 200, and 400 mg/kg. For tumor marker hormone tests, blood sampling was conducted after 10 days. Blood was collected from the hearts of the animals and centrifuged to separate the serum.
ZnO NPs synthesis method
The materials required for this purpose consisted of (1) Zn (NO3). (2) 6H2O. (3) NAOH and distilled water as solvent (all in high purity). We dissolved some definite amounts of (NO3)2.6H2O and (NAOH) in water and derived intended concentrations. Primary solutions were wholly clear. Zinc nitrate solution was slowly transferred to sodium hydroxide solution while stirring by a magnetic stirrer in an appropriate temperature. First, there was no sediment obviously, but subsequent addition of definite values of ingredients made the mixture gradually opaque and extra white sediments were formed that were not solved even by stirring. Obtained sediments were filtered and rinsed several times with distilled water and ethanol. These residues were dehydrated for 8 hours at 122°C. finally ZnO precursor was calcified at 122°C to achieve NPs (15).
Preparation of NPs suspension
To prepare a stock solution of nanoparticles, 10g of NP was suspended in 1 lit. It was Sterilized medium and for their adequate dispersion the ultrasound device (PARSONIC 7500s, PARS NAHAND ENGG Co .IRAN) was used for 30 minutes. For error avoiding the NPs suspension was prepared.
Data extraction procedure
The obtained data from electro-chemiluminescence device by employing SPSS software was stored safely. Obtained data from ANOVA chart was extracted from SPSS programs and entered into MS- EXCELL. The results were implicated statistically as mean and standard deviation. As for the normal distribution of data, to compare the enzyme results in each group prior and after analysis, ANOVA test with continuous evaluation and to compare groups in each periods ANOVA and DUNNETTS tests were used. Significant level was considered less than 0.05 (P<0.05).
Results
Electron microscopic analysis of ZnO NPs
Basic function of Transmission Electron Microscope (TEM) is similar to optical microscopes; except of the fact that instead of optical ray electron ray is used. Image resolution in TEM is 1000 times greater than an optical microscope so it is appropriate for measuring sizes of smaller features. Magnification is also multiple times greater in TEM compared to optical microscopes. TEM image of ZnO NPs has been shown in Fig.1. Due to the increase in ratio of surface to volume by decrease in NPs size, these are able to play a decent role in immobilization processes. According to the achieved results from TEM studies, the synthesized ZnO NPs’ diameter is in the range of 20-30 nm.
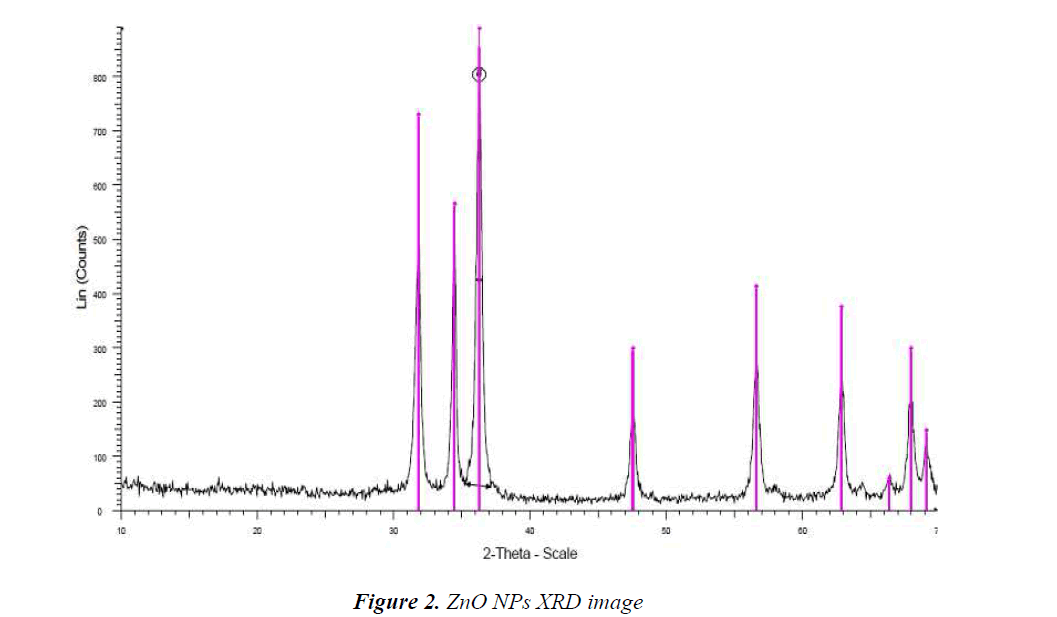
XRD pattern of produced NPs
X-Ray Diffraction (XRD) is an old and widely applied technique in studying properties of crystals. In this method, XRD of a sample is used to investigate the properties of a sample. XRD is applicable to specify crystalline structure such as lattice constant, lattice geometry, unknown materials quality identify, crystal phase determination, size measurement of crystals, single crystal bios, stress, tension, lattice faults, and so on. The XRD pattern shown in Fig.2 is for ZnO NPs and diffraction peaks have been absorbed in 2θ value. The highest peak was used to estimate the pattern size by Scherrer equation D = Kλ/ (β cos θ) in which; K is consonant (0.9), λ is wavelength (Cu Kα) (λ = 1.5418 A), β is full width at the half-maximum and θ is diffraction angle; the increase in sharpness of XRD peak tip refers to crystalline nature of NPs. All different peaks in Fig.2 represent ZnO NPs which; would be bound to study diffraction of powders.
Serum analysis
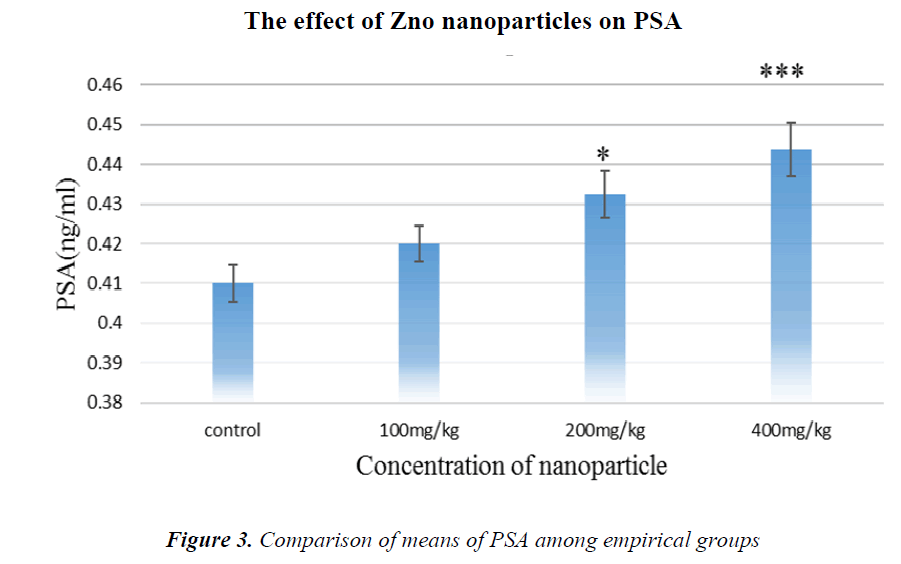
As it is shown in Fig.3, PSA tumor marker concentration has been increased in all groups compared to controls, but in the group receiving 400 mg/kg NPs, it is significant.
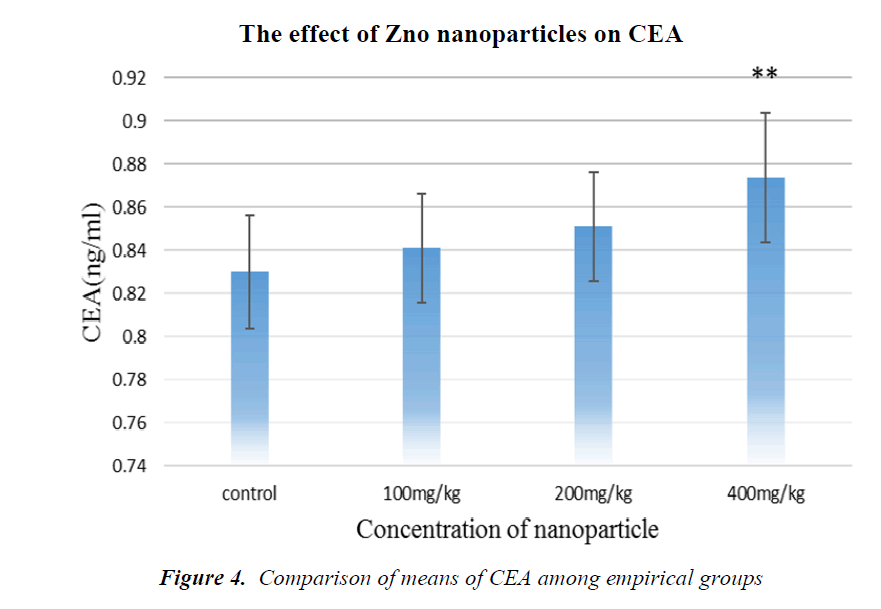
As it is shown in Fig. 4, CEA tumor marker concentration has been increased in all groups in comparison with the control, but in the group receiving 400 mg/kg NPs, it is significant.
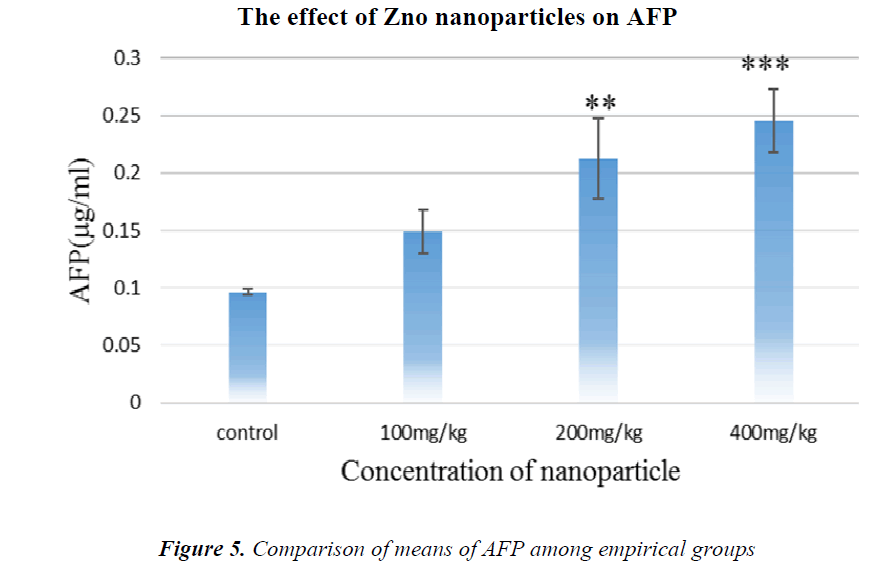
As it is shown in Fig.5, AFP tumor marker concentration has been increased in all groups in comparison with the control, but in the groups receiving 200 and 400 mg/kg NPs, it is significant.
Discussion
Today contaminations stemming from NPs are being considered as novel hazardous problems. Metal oxide NPs are long lasting in the environment and food chains are causing considerable toxics (1-3). Nowadays for early diagnosis of disease, prognostic, and in cancer treatment procedure, detection of variable proteins in individuals’ blood is more common. State-of-the-art methods of immunologic evaluation such as chemiluminescence, has higher precision and sensitivity compared to previous methods. Moreover, the detection of materials in blood is performed prior to Biopsy and Tissue analysis due to its non-invasive nature and reproducibility of results.
AFP is increased in liver diseases, but simultaneous use of AFP with sonography, promotes the accuracy of diagnosis up to 100%. CEA is a glycoprotein; which was earlier supposed to be specific for colon cancers only, but later it was found that it is also elevated in breasts, lungs, and liver cancers. CEA is very useful in specifying prognosis, recrudescence, and metastasis, and is also proposed for use in staging. PSA is used in annual screening for prostate cancer. Researches on spermatozoid cells have shown that in vitro Ag NPs can affect sperm fertility. Applying NPs in rats causes significant and dose-related increase in testosterone. Fuse et al. (1999) showed that there is a significant relationship between zinc concentration and plasma testosterone and sufficient zinc is essential for normal functioning of sperm. Other studies show that the deficiency of ZnO NPs results in atrophy of seminiferous tubules and impairment of spermatogenesis in rats, however in the present study using ZnO NPs we have observed an increase in tumor markers hormones. Carlson et al. (2008) concluded that NPs might affect the function of mitochondria in Leydig cells and thereby decrease its secretional activity. On the other hand, NPs may increase free oxygen levels such as superoxidase resulting in oxidation of molecules such as proteins. Production of radical hydroxyl may lead to development of ROS as a result of presence of NPs, and may inflict severe and heritable damage to DNA. For instance, chemical modifications in histones or other proteins which play a role in forming the structure of DNA, deform the spiral structure of DNA and expose it against any change.
Probably oxidative lesions have caused the increase in tumor markers hormone in this study. Mitochondria genome is significantly vulnerable to oxidative invasion. Agarwal et al. (2003) showed that the high level of ROS results in breakdown of interior and exterior cell membranes, and as a result, it leads to the release of protein cytochrome C and activation of cascade of events and agitation of apoptosis therefore, it mediates the apoptosis procedure of ROS.
Zinc is an essential trace element required in low quantities by the body, but in a higher quantity is toxic and is able to induce apoptosis or even necrosis. Zinc acts as a cofactor of more than 300 metallic-enzymes involved in metabolism of proteins, lipids, carbohydrates, transcription of DNA and protein synthesis. As DNA transcription is an important part in development of gametes, zinc is an essential element for reproduction.
Some evidences show that zinc acts as a purifier of super-oxides produced by defective spermatozoids or leucocytes in vivo. Other tests have shown that zinc is able to purify induced free radicals by different factors such as ionizing radiations and results in decrease in MDA quantity, thus, it has been known as an antioxidant with high protection potency. The most common combination of zinc is its oxide form (ZnO) which is preferred for two reasons: first because of its high zinc concentration and second, because it’s highly absorbed by the body and is bearable by the gastrointestinal system. Recently, ZnO NPs has been widely considered in animal studies. Different NPs are novel forms of materials with significant biologic properties and low toxic levels. It seems that they have high potential in trespassing the physiological barriers of body and its accessibility to specific target tissues.
Conclusion
According to the findings it can be concluded that the negative effects of NPs are more significantly compared to their positive ones. NPs increase free radical levels inside the cells and cause tissue damages. This study showed that different doses of ZnO NPs have negative effects on biomarkers.
Abbreviations
TEM- Transmission Electron Microscopy.
uv-vis- Visible-Ultraviolet spectrums.
Optical density (OD)
Ammonium Bromide surfactant (CTAB)
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
We would like to thank everyone who helped us in this study. This article was extracted from the thesis of ZAHRA AREFIAN and the expenses of this research was borne by the Corresponding Author.
References
- Nohynek GJ, Lademann J, Ribaud C, Roberts MS. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Critical reviews in toxicology. 2007; 37(3): 251-277.
- Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, et al. Understanding biophysico- chemical interactions at the nano-bio interface. Nature materials. 2009; 8(7): 543-557.
- Roco MCTNNI, Past PaFneLS, Iafrate G, Eds.; Taylor and Francis Press: Boca, Raton and London C, ; 2007, pp. 1-42.
- Jin T, Sun D, Su JY, Zhang H, Sue HJ. Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella Enteritidis, and Escherichia coli O157: H7. Journal of food science. 2009; 74(1): M46-M52.
- He L, Liu Y, Mustapha A, Lin M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiological research. 2011; 166(3): 207-215.
- Rasmussen JW, Martinez E, Louka P, Wingett DG. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert opinion on drug delivery. 2010; 7(9): 1063-1077.
- Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Particle and fibre toxicology. 2005; 2(1): 8.
- Lu S, Duffin R, Poland C, Daly P, Murphy F, Drost E, et al. Efficacy of simple short-term in vitro assays for predicting the potential of metal oxide nanoparticles to cause pulmonary inflammation. Environmental health perspectives. 2009; 117(2): 241-247.
- Kroll A, Pillukat MH, Hahn D, Schnekenburger J. Current in vitro methods in nanoparticle risk assessment: limitations and challenges. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2009; 72(2):370-377.
- Sharma V, Shukla RK, Saxena N, Parmar D, Das M, Dhawan A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicology letters. 2009; 185(3): 211-218.
- Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicological sciences : An official journal of the Society of Toxicology. 2007; 97(1): 163-180.
- Cho WS, Duffin R, Howie SE, Scotton CJ, Wallace WA, Macnee W, et al. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Particle and fibre toxicology. 2011; 8: 27.
- Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicology letters. 2007; 168(2): 176-185.
- Baek M, Chung HE, Yu J, Lee JA, Kim TH, Oh JM, et al. Pharmacokinetics, tissue distribution, and excretion of zinc oxide nanoparticles. International journal of nanomedicine. 2012; 7: 3081-3097.
- Chen C, Liu P, Lu C. Synthesis and characterization of nano-sized ZnO powders by direct precipitation method. Chemical Engineering Journal. 2008; 144(3):509-513.
- Li W, Hsiao G, Harris D, Nyffenegger R, Virtanen J, Penner R. Mechanistic study of silver nanoparticle deposition directed with the tip of a scanning tunneling microscope in an electrolytic environment. The Journal of Physical Chemistry. 1996; 100(51): 20103-20113.
- Nervey DW, Patric IS, Godwin AO. The Influence of Excessive and Prolonged Ingestion of Honey on Sex Hormones and Prostate Specific Antigen in Adult Male Wistar Rats. Medicine Science| International Medical Journal. 2012; 1(3): 161-170.
- Burd A, Kwok CH, Hung SC, Chan HS, Gu H, Lam WK, et al. A comparative study of the cytotoxicity of silver-based dressings in monolayer cell, tissue explant, and animal models. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2007; 15(1): 94-104.
- Fuse H, Kazama T, Ohta S, Fujiuchi Y. Relationship between zinc concentrations in seminal plasma and various sperm parameters. International urology and nephrology. 1999; 31(3): 401-408.
- Hammadeh ME, Filippos A, Hamad MF. Reactive oxygen species and antioxidant in seminal plasma and their impact on male fertility. Int J Fertil Steril. 2009; 3(3): 87-110.
- Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, et al. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. The journal of physical chemistry B. 2008; 112(43): 13608-13619.
- Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, et al. NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials. 2009; 30(23-24): 3891-3914.
- Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer research. 2009; 69(22): 8784-8789.
- Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertility and sterility. 2003; 79(4): 829-43.
- Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Human reproduction update. 2007; 13(2): 163-174.
- Klotz LO, Kroncke KD, Buchczyk DP, Sies H. Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. The Journal of nutrition. 2003; 133(5 Suppl 1): 1448S- 1451S.
- Favier AE. The role of zinc in reproduction. Hormonal mechanisms. Biological trace element research. 1992; 32: 363-382.
- Dani V, Dhawan DK. Radioprotective role of zinc following single dose radioiodine (131I) exposure to red blood cells of rats. The Indian journal of medical research. 2005; 122(4): 338-342.
- Hollis GR, Carter SD, Cline TR, Crenshaw TD, Cromwell GL, Hill GM, et al. Effects of replacing pharmacological levels of dietary zinc oxide with lower dietary levels of various organic zinc sources for weanling pigs. Journal of animal science. 2005; 83(9): 2123-2129.
- Hotz C, DeHaene J, Woodhouse LR, Villalpando S, Rivera JA, King JC. Zinc absorption from zinc oxide, zinc sulfate, zinc oxide + EDTA, or sodium-zinc EDTA does not differ when added as fortificants to maize tortillas. The Journal of nutrition. 2005; 135(5): 1102-1105.
- Rosado JL, Díaz M, Muñoz E, Westcott JL, González KE, Krebs NF, et al. Bioavailability of zinc oxide added to corn tortilla is similar to that of zinc sulfate and is not affected by simultaneous addition of iron. Food and nutrition bulletin. 2012; 33(4): 261.
- Dawei A, Zhisheng W, Anguo Z. Protective Effects of Nano-ZnO on the Primary Culture Mice Intestinal Epithelial Cells in in vitro Against Oxidative Injury. Journal of Animal and Veterinary Advances. 2009; 8(10): 1964-1967.