Research Article - Biomedical Research (2017) Volume 28, Issue 6
Effect of varenicline on colonic motility in a rat model of experimental irritable bowel
Tijen Kaya Temiz1*, Omer Demir2, Elif Keskin-Arslan1, Selin Acar1, Baris Karadas1, Gokhan Koyluoglu31Department of Pharmacology, Faculty of Medicine, Izmir Katip Celebi University, Izmir, Turkey
2Department of Pharmacology, Faculty of Medicine, Izmir University, Izmir, Turkey
3Department of Paediatric Surgery, Faculty of Medicine, Izmir Katip Celebi University, Izmir, Turkey
- *Corresponding Author:
- Tijen Kaya Temiz
Department of Pharmacology
Izmir Katip, Celebi University, Turkey
Accepted date: November 11, 2016
Abstract
Aim: We aimed to investigate in vitro effects of varenicline on spontaneous contractile responses of proximal and distal colon smooth muscle in control and IBS rats.
Methods: The animal model of IBS-like visceral hypersensitivity was induced by intra-colonic infusion of 0.5% Acetic Acid (AA) in saline once daily between postnatal days 8-21. Control animals received saline instead of AA. Experiments were performed at the end of the 8 weeks. Proximal and distal colon tissues were resected and concentration-dependent effects of varenicline were evaluated in vitro tissue bath.
Results: The mean pressure values of spontaneous contractions and KCL (80 mmol/L) responses of proximal and distal colonic segments were similar in control and IBS rats. Varenicline significantly decreased the mean pressure of spontaneous colonic contractions in both control and IBS rats compared to their baseline values in only distal colon (p<0.05). The relaxation responses of distal colons were not significantly different between IBS and control groups (p>0.05).
Conclusion: Varenicline decreased the mean pressure values of spontaneous colonic contractions only distal colon in both control and IBS rats. Varenicline may reduce the increased distal colonic response with direct effect, as well as improve partially the disrupted cholinergic response with in vivo partial agonist effect. Thus, varenicline may have a potential usage in IBS as an adjuvant.
Keywords
Irritable bowel syndrome, Varenicline, Colonic contractility, In vitro
Introduction
Irritable Bowel Syndrome (IBS) is a highly prevalent (from 5% to 20%) functional Gastrointestinal (GI) disorder that reduces patients’ quality of life [1,2]. The manifestations include abdominal discomfort, pain and changes in bowel habits. IBS have been classified into different subtypes like diarrhoea-predominant IBS, constipation-predominant IBS and diarrhoea-constipation concomitant IBS [3]. The pathophysiology of IBS is not definitely known yet because of multifactorial events such as genetic, immune, environmental, inflammatory, motility disorder, bacterial over growth, neurological and psychological factors, visceral hypersensitivity contribute. Gut-brain axis has an important role in the pathophysiology [1,4]. Bowel disorders may originate from abnormal motor function of the colon in IBS patients [5]. According to symptoms of patients; laxative drugs, anti-diarrheic drugs, serotonin receptor-3 (5-HT3) antagonists, serotonin receptor-4 antagonists (5-HT4) anti-spasmolytic anticholinergic drugs, antidepressant drugs, antiflatulents, antibiotics, probiotics, motility regulators can be used for treatment of IBS [4].
Varenicline is a selective partial agonist of the alpha 4 beta 2 (α4β2) nicotinic acetylcholine receptors (nAChRs) and approved in over 90 countries worldwide for the treatment of smoking cessation [6]. Varenicline affects dopamine cells which are present in mesolimbic system and responsible from nicotine addiction in this indication [7,8]. In addition, varenicline is also shown to be a partial agonist of α3β4, full agonist of α4β4 and α7 nAChRs [8-11].
GI motility is under the control of enteric nervous system, inflammatory mediators and diverse hormones [12]. The cholinergic transmission plays an important role in the regulation of gastrointestinal tract tonus and motility [13]. Acetylcholine receptors are expressed by many enteric nerves and facilitate enteric neurotransmission [12]. Anticholinergic agents show spasmolytic effects and reduce stomach ache and flatulence via inhibiting muscarinic acetylcholine receptors in IBS [4]. The presence of α3β4 nAChRs subtype on enteric nerves in myenteric plexus was demonstrated [14]. Also it is known that Non Adrenergic Non Cholinergic (NANC) system modulates motor functions of gastrointestinal tract [13]. Varenicline affects the α7 nAChRs as a full agonist. A recent study showed that the activation of α7 nAChRs suppresses in vitro hyperexcitability of colonic dorsal root ganglia neurons in a mouse model of acute colonic inflammation [15]. These findings suggest that varenicline may have a role on modulation of colonic motility.
Therefore, we aimed to investigate the possible direct effects of varenicline on spontaneous contractile responses in IBS rat colon. We hypothesized that; varenicline can improve the disrupted motor function of the colon in IBS through its modulatory effects on nicotinic receptors. For this aim, we measured and compared the dose-dependent changes with varenicline on spontaneous motor activity of proximal and distal colonic segments of IBS and control rats in vitro.
Materials and Methods
Animals
Experiments were performed on male Wistar-Albino rats in Experimental Animal Laboratory of Dokuz Eylul University. Rats were housed with ad libitum food and water in standard rodent cages at 22 ± 2°C in a 12 h light-dark controlled room. All male neonates used in experiment were housed per cage with 1 adult female rat until they were one month old. The study protocol was reviewed and approved by the Animal Ethics Committee of the Dokuz Eylul University.
Induction of IBS
Neonatal male Wistar-Albino rats (n=11) were randomly divided into 2 groups. Group 1 (n=5) received colonic infusion of 0.9% saline as the control group. Group 2 (n=6) received an infusion of 0.3 ml of 0.5% acetic acid solution in saline into the colon 2 cm from the anus once daily on postnatal 8-21 days. Rats were tested for sensitivity to colorectal distension on day 43 [16]. According to visceral hypersensitivity model, rats were followed until 8 weeks and experiments were conducted at the end of the 8 weeks.
Evaluation of visceral sensitivity
On the 43rd day of our study, it was recorded that the threshold degree induced visually identifiable contraction of the abdominal wall and body arching during rectal distention to evaluate visceral hypersensitivity. After 30 min of adaptation in small box (20 × 8 × 8 cm) rectal distention was performed using the 6F Fogarty arterial embolectomy catheter (Edwards Life sciences LLC, USA) in the descending colon (1 cm from the anal verge). Rectal distention was performed with increasing volumes of saline in increments of 20 μL, starting at 100 μL. For each measurement, the rats were given rectal distention for 20 s every 2 min. The measurements were repeated three times for accuracy, and the difference between replicate measurements was<20%.
Recording of colonic motor activities
Rats were sacrificed by cervical dislocation and 2 cm distal and proximal colonic segment was removed. Segments were placed in circular direction in a 20 ml tissue baths, filled with preaerated (95% O2 and 5% CO2) Krebs Bicarbonate Solution (KBS) at 37°C (KBS composition in mmol/L: NaCl, 120; KCl, 4.6; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 22; NaH2PO4, 1.14 and glucose 11.5). The upper end of the segments was tied to an isometric force displacement transducer (FDT-05, MAY, Turkey) and preloaded with 0.6 g tension. Tissues were allowed to equilibrate for 30 min and washed at every 10 minutes.
After equilibrium, varenicline (10-6 and 10-5 mol/L, Sigma, St Louis, MO) was added cumulatively to the tissue bath to investigate the direct effect on distal and proximal colonic segments in both control and IBS rats. Varenicline was prepared freshly on the day of the experiment.
Firstly, the mean pressure values of spontaneous colonic contractions were measured at each segment in both control and IBS groups. And then, direct effects of cumulative concentrations of varenicline were calculated as a change of the mean pressure of the initial spontaneous colonic contraction.
At the end of all experiments, the tonic contraction by KCl (80 mmol/L) was measured to test the contraction health of colonic smooth muscle isolated from the control and IBS groups.
Statistical analysis
Statistical analyses were performed using SPSS 22.0 software demo (IBM Software Corp., NY). All data were expressed as mean ± standard error of mean (SEM). The normality of distribution was assessed by Shapiro-wilk test, skewness and kurtosis of the data. We used non-parametric tests for group comparisons as the normality assumption of the data was violated in our case. Statistical comparisons for repeated measurements within groups were evaluated by Friedman test followed by Wilcoxon signed rank tests to determine the significant group. Comparisons between groups were evaluated with Mann Whitney U test. Differences were considered to be significant when p<0.05.
Results
Response to visceral sensitivity
We observed visceral hypersensitivity in adult rats. Rats were tested for sensitivity to colorectal distention on day 43. Threshold of abdominal wall contraction and body arching in response to increasing colorectal distension was significantly lower in the acetic acid (n=6) group than in the control (n=5) group (0.21 ± 0.06 vs. 0.42 ± 0.19 ml for abdominal muscle contraction and 0.43 ± 0.13 vs. 0.85 ± 0.24 ml for body arching, P<0.01, t-test) There was no difference in body weight between control and IBS groups.
Effect of varenicline on spontaneous motor activities of isolated distal and proximal colonic segments
The isolated colonic segments showed spontaneous motor activities in rest. The spontaneous contractile activity of the distal and proximal colon did not significantly change in IBS rats compared to control rats. The mean pressure values of the spontaneous distal colonic contractions were 443.60 ± 265.89 mmHg in control (n=5) and 410.83 ± 262.88 mmHg in IBS (n=6) rats, respectively (p>0.05). The mean pressure values of the spontaneous proximal colonic contractions were 593.60 ± 201.94 mmHg in control (n=5) and 507.83 ± 251.48 mmHg in IBS (n=6) rats, respectively (p>0.05).
Contractions of proximal and distal colonic segments isolated from control and IBS rats were recorded by transducer as traces (Figure 1). Spontaneous motor contractions of proximal colon did not change at 10-6 and 10-5 mol/L concentrations of varenicline in both control (Figure 1A) and IBS (Figure 1B) rats. Spontaneous motor contractions of distal colon progressively decreased at 10-6 and 10-5 mol/L concentrations of varenicline in both control (Figure 1C) and IBS (Figure 1D) rats.
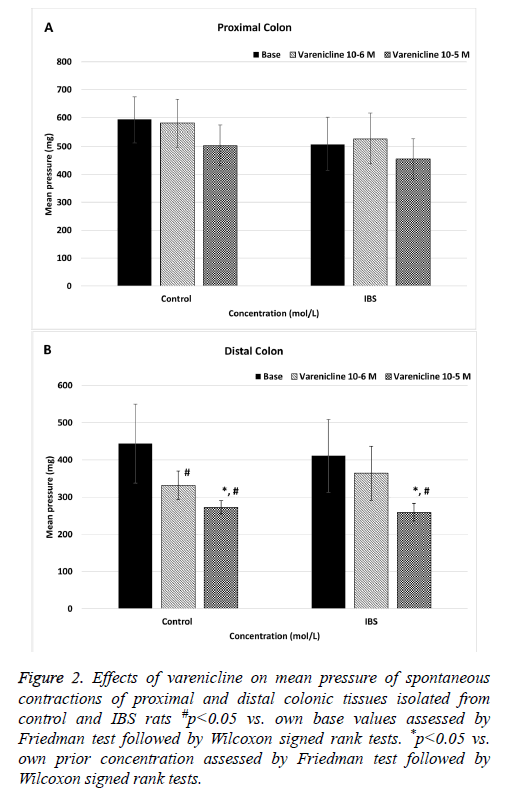
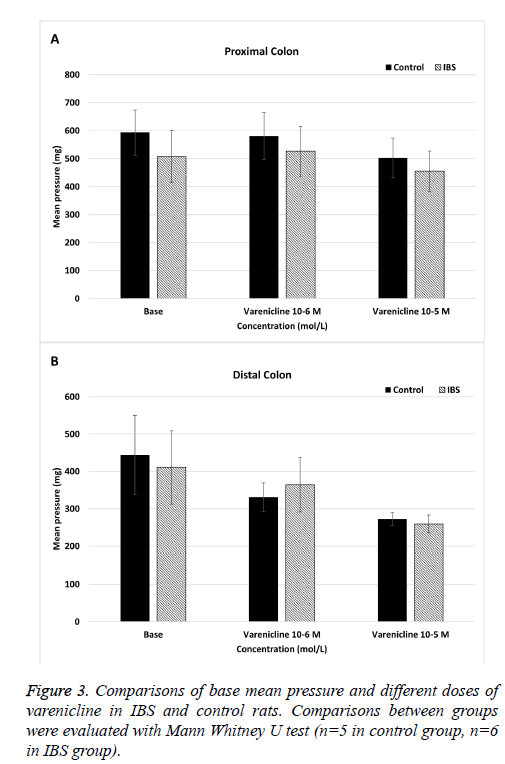
The mean pressure values of the spontaneous proximal colonic contractions did not significantly change in both control and IBS rats when varenicline was administered at concentration of 10-6 and 10-5 mol/L (p>0.05) (Figure 2A). When IBS rats compared to control rats, the mean pressure values of the spontaneous proximal colonic contractions were not significantly different at cumulative doses of varenicline (p>0.05) (Figure 3A).
Figure 2: Effects of varenicline on mean pressure of spontaneous contractions of proximal and distal colonic tissues isolated from control and IBS rats #p<0.05 vs. own base values assessed by Friedman test followed by Wilcoxon signed rank tests. *p<0.05 vs. own prior concentration assessed by Friedman test followed by Wilcoxon signed rank tests.
Cumulative doses of varenicline (10-6 and 10-5 mol/L) significantly decreased the mean pressure values of spontaneous distal contractions in control rats (p<0.05) (Figure 2B). In IBS rats, although the mean pressure values of the spontaneous distal colonic contractions did not significantly change at varenicline concentration of 10-6 mol/L (p>0.05), it significantly decreased at concentration of 10-5 mol/L (p<0.05) (Figure 2B). Also there was a statistically significant difference in mean pressure values between two concentrations (10-6 and 10-5 mol/L) of varenicline (p<0.05) (Figure 1B). When IBS rats compared to control rats, there was not significantly difference in the mean pressure values of the spontaneous distal colonic contractions at all doses of varenicline (p>0.05) (Figure 3B).
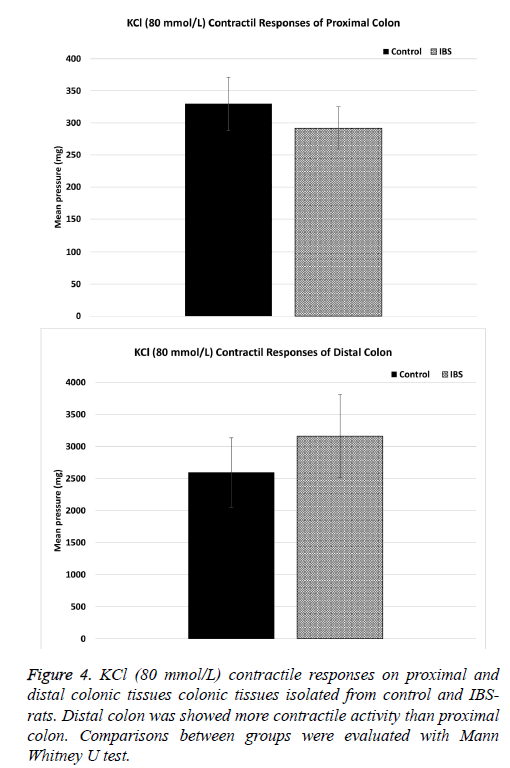
The contractile responses to KCl (80 mmol/L) in distal and proximal colon, which were tested at the end of each experiment, did not significantly different between control (n=5) and IBS (n=6) groups. KCl contractile responses were 329.68 ± 43.67 and 291.61 ± 35.01 in control (n=5) and IBS (n=6) rats in proximal colon, respectively (p>0.05) (Figure 4A). KCl contractile responses were 2592.27 ± 569.87 and 3161.98 ± 671.64 in control (n=5) and IBS (n=6) rats in distal colon, respectively (p>0.05) (Figure 4B).
Discussion
IBS is a functional bowel disorder of which major clinical symptom is disordered defecation associated with abdominal pain/discomfort [3]. According to the predominant bowel habit, IBS is classified into different subtypes clinically as IBS with diarrhoea, IBS with constipation and alternating form [17]. Pathogenesis of IBS has not been clarified completely [17]. However when the changing motor function of colon in IBS is considered, it's possible that cholinergic receptors which is commonly in the enteric nerve system have a role.
Varenicline is a ligand of nAChRs [8], the ligand-gated pentameric ion channels which is formed by the combination of alpha and non-alpha subunits as heteromeric and homomeric pentamer [14]. The previous studies showed that varenicline was a partial agonist of α4β2 and α3β4, and full agonist of α7 and α4β4 nAChRs [8-11]. Garza et al. demonstrated that the predominant subunits of nAChRs in rat neurons of the myenteric and submucosal plexus are formed by α3, α5 and β4 subunits, which is similar to the peripheral ganglia [14]. David et al. showed the predominant presence of α3β4 nAChRs in wild type mice cervical ganglion [18]. Therefore, it is possible that the stimulation of these receptors by varenicline may change the colonic motor response. Several studies indicated that the stimulation of nAChRs in myenteric excitatory motor nerve leads to contraction of circular smooth muscle [19,20]. In enteric nerve system, inhibitor neuromediators released from NANC nerve endings are adenosine, ATP, Vazoactive Intestinal Peptid (VIP), substance P and Nitric Oxide (NO) [13]. The stimulation of nAChRs increases the colonic motility and contractions [21]. However, neuromediators released from NANC nerve endings may reduce the colonic contractions.
In the present study, the animal model of IBS-like visceral hypersensitivity was induced by intra-colonic infusion of 0.5% acetic acid. And then, the direct effects of varenicline on proximal and distal colonic segments were evaluated in vitro tissue bath and results were compared to control rats. Varenicline did not significantly change the proximal colon response in both control and IBS rats. This result suggests that nicotinic receptors may not enough amount and/or mechanisms which mediates the direct effects of varenicline may not available or working to result in a significant effect in proximal colon. Varenicline significantly decreased the mean pressure values of distal colon according to initial spontaneous colonic activity in both control and IBS rats. The concentration which produced a significant effect was ten times higher in IBS rats. However, a statistically significant difference was not detected in varenicline response of distal colon in IBS group compared to control group. Our study is the first study which showed the direct effect of varenicline on colon smooth muscle in both control and IBS rats.
A contraction response in colon smooth muscle via direct effect was expected since varenicline is a partial agonist of α3β4 nAChRs. In contrast, it reduced the mean pressure values of colon in both groups. Grider et al. detected that Dimethylphenylpiperazinium (DMPP), nicotinic receptors agonist, leads to increase the releasing of L-citrulline (reflect NO production) and VIP in cavy ileum myenteric ganglion [22]. The stimulation of ganglion cells by DMPP was increased the syntheses of NO and NO increased the VIP secretion through cGMP-protein kinase [22]. In another study, intrinsic nerves of colon were actuated by the DMPP and relaxation was detected on colonic activity. Also relaxation was antagonized by the nerve blocker tetrodotoxin or the nicotinic receptor antagonist hexamethonium. The inhibition of ATP-sensitive K+ channels or blockage of P2y purinoceptors or inhibition of NO synthase antagonised the effect of DMPP [23]. Mean pressure decreasing effect of varenicline in distal colon smooth muscle both control and IBS rats, likewise DMPP, may be related to increased production and/or secretion of NO/cGMP or VIP via agonist effect of α3β4 nAChRs.
In this study, varenicline reduced the mean pressure value of distal colon at higher concentration in IBS rats. It may be associated with a disruption regarding the production and/or secretion of NO/cGMP and/or VIP in IBS rats. Grider et al. determined that NO production was suppressed by the ganglion blocker and the nicotinic receptor antagonist hexamethonium [22]. Findings of Grider et al. supports that nicotinic receptors may have an important role on NO production. In our previous study, we demonstrated that nNOS (neuronal NO-synthase) immunoreactivity reduced in IBS rats [24]. The inhibitory effect of varenicline was slightly decreased in distal colon of IBS rats. These findings may be related to decrease of NO amounts on colonic smooth muscle.
In our study, KCl contractile responses of colonic smooth muscle were not significantly different between the 2 groups in both distal and proximal colon. These results indicate that the contractile mechanisms of the colonic tissues were intact in both the control and IBS rats.
A partial agonist has two different effects which depend according to the concentration of the relevant endogenous neurotransmitter. If endogenous neurotransmitter presences high concentration in tissue, partial agonist reduces the effect of endogenous neurotransmitter. If endogenous neurotransmitter presences low concentration in tissue, partial agonist increases the effect of endogenous neurotransmitter [25]. When varenicline, α4β2 and α3β4 nAChRs partial agonist, is used for smoking cessation, patients have some adverse effects such as diarrhoea and constipation [26]. This situation is indicating that partial agonist varenicline may affect the bowel motor functions and be used in treatment of IBS symptoms which characterize altered bowel movements.
In conclusion, varenicline decreased the mean pressure value of distal colon in both control and IBS rats. The mean pressure value in IBS rats were significantly decreased with higher concentrations of varenicline compared to the control rats. Varenicline may reduce the increased distal colonic response with direct effect, as well as leading to a partial improvement in the disrupted cholinergic response with in vivo partial agonist effect. Varenicline may help to modulate the changing motility in IBS, since it is a partial agonist and it may be used in IBS treatment as an alternative drug. Further detailed studies are needed to determine the exact mechanism of effect of varenicline.
Acknowledgements
We are also grateful to Melih Kaan Sozmen for contribution regarding statistical analysis. The present research was supported by Izmir Katip Celebi University of Scientific Research Projects Coordination Unit as ONAP project, No. 213-3-TSBP-22.
Conflict of Interest
The authors report no conflict of interest.
References
- Bellini M, Gambaccini D, Stasi C, Urbano MT, Marchi S, Usai-Satta P. Irritable bowel syndrome: a disease stil searching for pathogenesis, diagnosis and therapy. World J Gastroenterol 2014; 20: 8807-8820.
- Lazaraki G, Chatzimavroudis G, Katsinelos P. Recent advances in pharmacological treatment of irritable bowel syndrome. World J Gastroenterol 2014; 20: 8867-8885.
- La JH, Kim TW, Sung TS, Kim HJ, Kim JY. Increase in neurokinin-1 receptor-mediated colonic motor response in a rat model of irritable bowel syndrome. World J Gastroenterol 2005; 11: 237-241.
- Unal HU. Irritablbarsaksendromu. GuncelGastroenteroloji 2012; 16: 213-217.
- Snape WJ Jr, Carlson GM, Matarazzo SA, Cohen S. Evidence that abnormal myoelectrical activity produces colonic motor dysfunction in the irritable bowel syndrome. Gastroenterology 1977; 72: 383-387.
- Faessel HM, Obach RS, Rollema H, Ravva P, Williams KE. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. ClinPharmacokinet 2010; 49: 799-816.
- Xi ZX. Preclinical pharmacology, efficacy and safety of varenicline in smoking cessation and clinical utility in high risk patients. Drug Healthc Patient Saf 2010; 2010: 39-48.
- Crunelle CL, Miller ML, Booij J, van den Brink W. The nicotinic acetylcholine receptor partial agonist varenicline and the treatment of drug dependence: a review. EurNeuropsychopharmacol 2010; 20: 69-79.
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. MolPharmacol 2006; 70: 801-805.
- Arias HR, Feuerbach D, Targowska-Duda K, Kaczor AA, Poso A, Jozwiak K. Pharmacological and molecular studies on the interaction of varenicline with different nicotinic acetylcholine receptor subtypes. Potential mechanism under lying partial agonism at human a4ß2 and a3ß4 subtypes. BiochimBiophysActa 2015; 1848: 731-741.
- Cippitelli A, Wu J, Gaiolini KA, Mercatelli D, Schoch J. AT-1001: a high-affinity α3β4 nAChR ligand with novel nicotine-suppressive pharmacology. Br J Pharmacol 2015; 172: 1834-1845.
- Rychter J, Espin F, Gallego D, Vergara P, Jimenez M, Clave P. Colonic smooth muscle cells and colonic motility patterns as a target for irritable bowel syndrome therapy: mechanisms of action of otilonium bromide. TherAdvGastroenterol 2014; 7: 156-166.
- Ergun Y. EnterikSinirSistemi: NitrikOksitveVazoaktif Intestinal PolipeptidArasindakiIliski. Arch Med Rev J 2007; 16: 111-144.
- Garza A, Huang LZ, Son JH, Winzer-Serhan UH. Expression of nicotinic acetylcholine receptors and subunit messenger RNAs in the enteric nervous system of the neonatal rat. Neurosci 2009; 158: 1521-1529.
- Abdrakhmanova GR, AlSharari S, Kang M, Damaj MI, Akbarali HI. (alpha)7-nAChR-mediated suppression of hyperexcitability of colonic dorsal root ganglia neurons in experimental colitis. Am J PhysiolGastrointest Liver Physiol 2010; 299:761-768.
- Yan C, Xin-Guang L, Hua-Hong W, Jun-Xia L, Yi-Xuan L. Effect of the 5-HT4 receptor and serotonin transporter on visceral hypersensitivity in rats. Braz J Med Biol Res 2012; 45: 948-954.
- Paterson WG, Thompson WG, Vanner SJ, Faloon TR, Rosser WW, Birtwhistle RW, Morse JL, Touzel TA. Recommendations for the management of irritable bowel syndrome in family practice. IBS Consensus Conference Participants. CMAJ 1999; 161: 154-160.
- David R, Ciuraszkiewicz A, Simeone X, Orr-Urtreger A, Papke RL, McIntosh JM, Huck S, Scholze P. Biochemical and functional properties of distinct nicotinic acetylcholine receptors in the superior cervical ganglion of mice with targeted deletions of nAChR subunit genes. Eur J Neurosci 2010; 31: 978-993.
- Galligan JJ, North RA. Pharmacology and function of nicotinic acetylcholine and P2X receptors in the enteric nervous system. NeurogastroenterolMotil 2004; 16: 64-70.
- Schneider DA, Perrone M, Galligan JJ. Nicotinic acetylcholine receptors at sites of neurotransmitter release to the guinea pig intestinal circular muscle. J PharmacolExpTher 2000; 294: 363-369.
- Carbone SE, Dinning PG, Costa M, Spencer NJ, Brookes SJ, Wattchow DA. Ascending excitatory neural pathways modulate slow phasic myogenic contractions in the isolated human colon. Neurogastroenterol Motil 2013; 25: 670-676.
- Grider JR, Jin JG. Vasoactive intestinal peptide release and L-citrulline production from isolated ganglia of the myenteric plexus: evidence for regulation of vasoactive intestinal peptide release by nitric oxide. Neurosci 1993; 54: 521-526.
- Borjesson L, Nordgren S, Delbro DS. DMPP causes relaxation of rat distal colon by a purinergic and a nitrergic mechanism. Eur J Pharmacol 1997; 334: 223-231.
- Temiz TK, Demir O, Simsek F, Kaplan YC, Bahceci S. Effect of nitrergic system on colonic motility in a rat model of irritable bowel syndrome. Indian J Pharmacol 2016; 48: 424-429.
- Hogg RC, Bertrand D. Partial agonists as therapeutic agents at neuronal nicotinic acetylcholine receptors. BiochemPharmacol 2007; 73: 459-468.
- Varenicline. Drugs.com 2016.