Research Article - Current Pediatric Research (2021) Volume 25, Issue 8
Effect of surfactant replacement therapy in preterm with respiratory distress syndrome in low sources center Al-Zahraa teaching Hospital at Al-Najaf city-Iraq.
Qassim Mohammed Hashim, Ameer Isam Al Aasam, Hala Mohsen Obeid Alwan and Alaa Jumaah Nasrawi*
Department of Pediatrics, College of Medicine, University of Kufa, Najaf, Iraq
- Corresponding Author:
- Alaa Jumaah
Nasrawi
Department of Pediatrics
University of Kufa
College of Medicine
Najaf
Iraq
Tel: 9647813088044
E-mail: alaaj.nasrawi@uokufa.edu.iq
Accepted date: August 15th, 2021
Abstract
Background: Every year, 15 million premature babies are born around the world. Prematurity and its complications cause more than a million deaths each year, accounting for approximately a third of all newborn deaths globally. Respiratory failure due to Hyaline Membrane Disease (HMD) is one of the primary causes of preterm infant death and morbidity. Aims of the study: 1) To assess the outcome of surfactant therapy using INSURE method in the management of respiratory distress syndrome. 2) To define the cofounder that may associate with its failure. Methods: This is a cross sectional study was performed in Al Zahraa teaching hospital for maternity and children in a period between January till June 2021. All neonates (gestational age between 28-32 weeks) were enrolled in the study. All of them have been diagnosed as RDS depending on clinical signs (tachypnea, grunting, nasal flaring, and cyanosis, substernal and inter costal retractions) and radiological features (air bronchograms, increasing hypo-aeration, reticulogranular or ground glass opacification). All of them were included in this experiment after receiving SRT using the INSURE technique. The required dose of surfactant was drawn out from its ampoule into a sterile syringe and allowed to warm to room temperature. After endotracheal tube inserted the infant is positioned supine on a flat surface with head turned to one side. Results: A total number of 50 neonates with clinical and radiological signs of RDS were included in the study; 54% of the study was male compared to female 46%. INSURE method was successful in saving the life of 31 out of 50 (62%) of them. The mean birth weights in the failure group were 1180(± 334) grams was significantly lower than the success group 1485 (± 428) grams. This study also showed that the INSURE method was successful in 26 among 36(72%) neonates with gestational age ≥ 30 weeks while succeed in 4 among 14(28%) neonates with gestational age <30 weeks. The severity of RDS was significantly increased in the failure group than in success group (88.8% vs. 12.5%). Tachycardia is most common complication during surfactant replacement therapy (36%), bradycardia (28%) and de saturation (26%). Conclusion: INSURE method is good method in management of respiratory distress syndromethat associated with decreased need for mechanical ventilation & decreased neonatal mortality rate. This study shows that preterm neonates with lower birth weight, lower gestational age, lower Apgar score at 5 minutes, with severe RDS & with prolonged administration have increased risk for INSURE method failure. Tachycardia, bradycardia and de saturation are the main complications during surfactant giving. And enough ICU beds with good nursing care appropriate with number improving outcomes of preterm patient. Every patient was received surfactant echocardiogram must be done to him for risk of PDA.
Keywords
RDS, Surfactant, Neonates, Hyaline membrane disease.
Introduction
Every year, 15 million premature babies are born around the world. Prematurity and its complications cause more than a million deaths each year, accounting for approximately a third of all newborn deaths globally [1]. Respiratory failure due to Hyaline Membrane Disease (HMD) is one of the primary causes of preterm infant death and morbidity, with severity inversely proportional to gestational age [2]. In Low and Middle Income Countries (LMICs), mortality rate ranges from 57 to 89 percent [3]. RDS adds to mortality indirectly by raising the risk of intra-ventricular hemorrhage, bronchopulmonary dysplasia [4]. In premature infants, RDS is caused by a pulmonary surfactant deficit. Its risk rises with decreasing gestational age, with a 60 percent chance in the first 28 weeks and a 30% chance between 28 and 34 weeks. Using a conservative estimate of 1% of all live births as the incidence of RDS, roughly 1.4 million neonates develop RDS each year, (137 688 million live birth per year around world) [5].
Pathophysiology
Surfactant deficit, especially in the context of underdeveloped lungs, causes neonatal respiratory distress syndrome. Surfactant insufficiency increases surface tension in the tiny airways and alveoli, limiting lung compliance in the immature stage. To prevent the alveolus from collapsing or filling with fluid, a careful balance of pressures at the air-fluid interface is required. P=2T/R, where P is pressure, T is surface tension, and R is the radius can be used to represent the pathophysiology of RDS. The relationship between the pressure differences across the interface of two static fluids and the geometry of the surface is described by laplace law. The amount of pressure necessary to preserve alveolar form increases as surface tension increases at the alveolar level. Atelectasis develops throughout the lung as a result of decreased surfactant synthesis, resulting in decreased gas exchange. The respiratory epithelium is eventually damaged by widespread and frequent atelectasis, resulting in a cytokinemediated inflammatory response. As the inflammatory reaction causes pulmonary edema, increasing amounts of protein-rich fluid from the vascular space flow into the alveoli, further inactivating surfactant [6,7]. In addition, many newborns with RDS require mechanical ventilation, which has the potential to harm the lungs. During positive pressure breathing, excessive distension of the alveoli causes further injury and inflammation. Furthermore, stressful oxidation caused by high increasing of oxygen tensions from mechanical ventilation and many inflammatory processes in the lung promotes the transformation of surfactant into an inactive form via protein oxidant damage and lipid peroxidation [8-10]. As a result, RDS can lead to hypoxemia through alveolar hyperventilation, diffusion abnormality, ventilation-perfusion mismatch, and Shunting between the lungs. Increased anaerobic metabolism at the cellular level, with lactic academia as a result, is this caused by of hypoxemia and tissue hypo perfusion.
Risk factors of RDS [11]:
• Premature delivery.
• Male infants.
• Infants delivered via caesarean section without maternal labour.
• Hypothermia.
• Perinatal asphyxia.
• Maternal diabetes.
• Family history of IRDS.
Clinical features of RDS [12]:
• Tachypnea
• Grunting
• Nasal flare-ups
• Bluish discoloration of skin and mucous membranes
• Retractions of the sub sternum and intercostals
Radiological features
Reticulogranular or ground glass opacification, increasing hypo aeration, and air bronchograms (Radiological abnormalities are highly related with clinical severity). Over the first 6 hours of life the symptoms and radiological symptoms increase, and granular densities persist for 3-5 days, clearing from peripheral to central and upper to lower lungs [13].
Grading of distress severity
Distress severity grading: Silverman-Anderson and Downes' scores are used to determine the severity of respiratory distress. While the SilvermanAnderson retraction score is more suited to preterm infants with HMD, the Downes' score is more extensive and can be used for any gestational age and condition. Half-hourly intervals should be scored, and a progress chart should be kept. (Tables 1 and 2) [14,15]. Is also a sensitive indicator of the severity and progress of distress. The majority of resource-poor countries lack laboratory facilities at most hospitals the diagnosis of RDS depends on clinical assessment [16].
| Score | Upper chest retraction | Lower chest retraction | Xiphoid retraction | Nasal dilation | Grunt |
|---|---|---|---|---|---|
| 0 | Synch | None | None | None | None |
| 1 | Lag on inspiration | Just visible | Just visible | Minimal | Stethoscope only |
| 2 | See-saw | Marked | Marked | Marked | Naked ear |
Table 1. Silverman Anderson retraction score. A score of >6 is indicative of impending respiratory failure.
| Score | Respiratory rate | Cyanosis | Air entry | Grunt | Retraction |
|---|---|---|---|---|---|
| 0 | <60/min | Nil | Normal | None | Nil |
| 1 | 60-80/min | In room air | Mild? | Ausc with stethoscope | Mild |
| 2 | >80/min | In >40% | Marked? | Audible with naked ear | Moderate |
Table 2. Downes’ score. A score of >6 is indicative of impending respiratory failure. A score of >6 is indicative of impending respiratory failure.
Treatment
Continuous Positive Airway Pressures (CPAP) and surfactant are two methods that have changed the treatment of Respiratory Distress Syndrome (RDS). At the conclusion of expiration, CPAP stabilizes the alveoli and preserves the function of endogenous surfactant [17]. It has been proven to lower infant mortality and the requirement for oxygen therapy by about a third, as well as the need for surfactant, by half [18,19].
Surfactant
By the early 1990s, surfactant replacement had been recognized as an effective and secure treatment for surfactant deficiency in preterm infants. 1 the incidence of pulmonary air leak (pneumothoraxes and pulmonary interstitial emphysema) became lowers and lowers mortality after surfactant administration in preterm infants with established respiratory distress syndrome that was confirmed by systematic reviews of randomized, controlled trials. Subsequent tests have shown that prophylactic or early administration of surfactant resulted enhanced survival without Broncho-Pulmonary Dysplasia (BPD), decreased pneumothoraxes, and decreased pulmonary interstitial emphysema and reduced risk of death at 28 days of age. Recent randomized clinical trials, however, have indicated that, when continuous positive airway pressure is routinely used, the advantages of prophylactic surfactant are no longer evident for babies in groups [20].
Aims of the study
• To assess the outcome of surfactant therapy using INSURE method in the management of respiratory distress syndrome.
• To define the cofounder that may associate with its failure.
Materials and Methods
This is a cross sectional study was performed in Al Zahraa teaching hospital for maternity and children in a period between months of January till June 2021. A total number of 50 premature neonates (gestational age between 28-32 weeks) were enrolled in the study. All of them have been diagnosed as RDS depending on clinical signs (tachypnea, grunting, nasal flaring, and cyanosis, substernal and intercostal retractions) and radiological features (air bronchograms, increasing hypoaeration, reticulogranular or ground glass opacification) All of them were included in this experiment after receiving SRT using the INSURE technique.
Other preterm neonates were excluded according to the following exclusion criteria
• Diagnosis other than RDS (transient tachypnea of newborn, congenital pneumonia, meconium aspiration, diaphragmatic hernia, congenital heart disease, birth asphyxia).
• The presence of congenital anomalies incompatible with life beyond the neonatal period.
• Patient hemodynamically unstable.
• Active pulmonary hemorrhage.
According to these inclusion and exclusion criteria; a total number of 50 neonates had been enrolled in the study. Neonatal variables (gender, birth weight, gestational age, respiratory distress syndrome severity and the severity of respiratory distress syndrome on the initial chest X-ray) had been evaluated before INSURE method.
The neonatal variables after INSURE method which include the need for 2nd dose of surfactant, duration of CPAP treatment after surfactant therapy and complication during the course of respiratory distress syndrome has been evaluated as well. According to Downe’s score criteria was used to assess the severity of RDS into mild, moderate & severe. Following the initial steps of resuscitation 3 different approaches are available for management of RDS:
• Respiratory support: No sign of increase work of breathing and not require any supplemental oxygen within 6 hrs. Of resuscitation; these neonates monitored for the development of any signs of respiratory distress then INSURE method will be initiated.
• INSURE method: For those infants with signs of moderate to severe respiratory distress and confirmed by radiological findings at 30 min or later and their oxygen saturation (SpO2) <85% in room air, they were intubated electively and given surfactant (dose 100 mg/kg) then immediately assessed for extubation if they have good respiratory drive and clinically stable. Then put on CPAP.
• Mechanical ventilation: For those with poor respiratory drive following surfactant delivery or clinically unstable following resuscitation.
Outcome Evaluation
• FIO2 requirements are reduced.
• Breathing effort is reduced.
• A chest x-ray shows an improvement in aeration.
• Increased lung volume and improved pulmonary mechanics (compliance, airway resistance) (functional residual capacity).
• Decreased ventilator support (peak inspiratory pressure, positive end expiratory pressure, and airway pressure).
INSURE method considered to be successful if the neonate survived. The statistical analysis was done by SPSS version 20 from IBM. P-value was considered positive when it is <0.05 by T test and Chi square.
Results
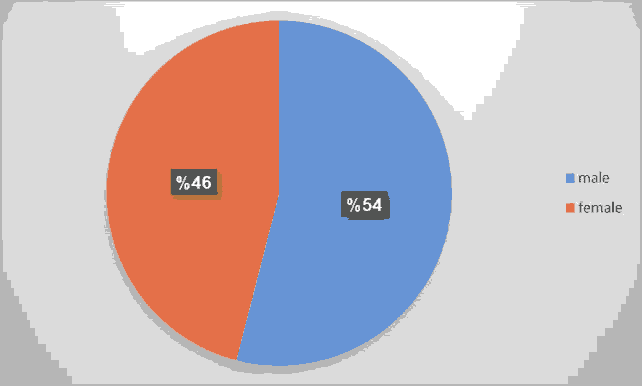
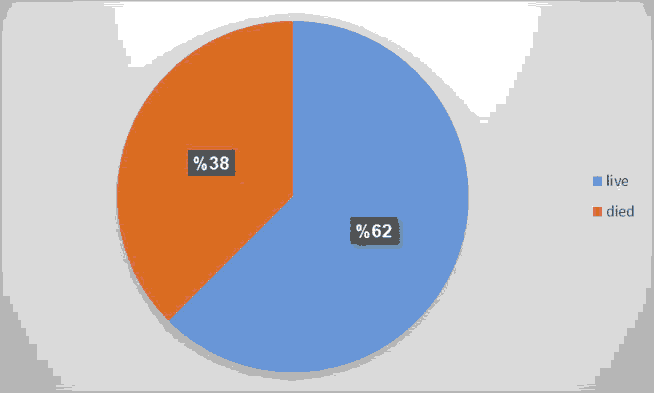
A total number of 50 neonates with clinical and radiological signs of RDS were included in the study; 54% of them were male. INSURE method was successful in saving the life of 31 out of 50 (62%) of them (Figures 1-3 and Tables 3-7).
| Outcome | Total | P value | |||
|---|---|---|---|---|---|
| Live | Died | ||||
| GA | <30 | 4 | 10 | 14 | 0.005 |
| (wks) | ≥ 30 | 26 | 10 | 26 | |
| Total | 30 | 20 | 50 | ||
| Gender | Total | P value | |||
| male | female | ||||
| Outcome | Live | 18 | 12 | 30 | 0.297 |
| Died | 9 | 11 | 20 | ||
| Total | 27 | 23 | 50 | ||
| Mode of delivery | Total | P value | |||
| C/S | NVD | ||||
| Outcome | Live | 22 | 8 | 30 | 0 |
| Died | 3 | 17 | 20 | ||
| Total | 25 | 25 | 50 | ||
| Antenatal steroid | Total | P value | |||
| YES | NO | ||||
| Outcome | Live | 18 | 12 | 30 | 0.083 |
| Died | 7 | 13 | 20 | ||
| Total | 25 | 25 | 50 | ||
Table 3. Shows comparison between live and dead neonates who received surfactant by INSURE method according to multiple pre-natal neonatal variables.
| Outcome | N | Mean | Std. Deviation | P value | |
|---|---|---|---|---|---|
| Birth weight | live | 30 | 1485 | 428.54 | 0.01 |
| (gm) | died | 20 | 1180 | 334.978 | |
| Apgar 1 | live | 30 | 4.1 | 0.994 | 0 |
| died | 20 | 2.6 | 1.535 | ||
| Apgar 2 | live | 30 | 7.566 | 0.817 | 0.003 |
| died | 20 | 6.7 | 1.128 | ||
| Duration of CPAP (Hrs.) | live | 30 | 37.23 | 18.786 | 0.315 |
| died | 20 | 31.5 | 20.73 | ||
| RDS severity | live | 30 | 2.066 | 0.253 | 0 |
| died | 20 | 2.8 | 0.41 |
Table 4. Shows comparison between live and dead neonates who received surfactant by INSURE method according to multiple post-natal neonatal variables.
| Outcome | Total | P value | |||
|---|---|---|---|---|---|
| Lives | Died | ||||
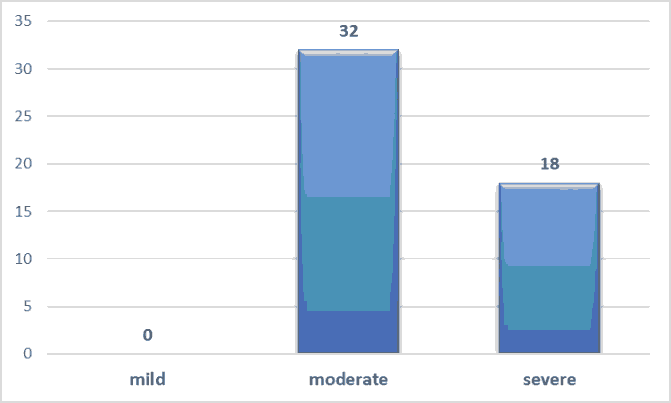
| RDS severity | moderate | 28 | 4 | 32 | 0 |
| severe | 2 | 16 | 18 | ||
| Total | 30 | 20 | 50 | ||
Table 5. Showing the association between RDS severity and the outcome of neonates treated by INSURE method.
| Outcome | Total | P value | |||
|---|---|---|---|---|---|
| Live | Died | ||||
| Time of giving surfactant | ≤ 1 hr | 21 | 7 | 28 | 0.015 |
| > 1 hr | 5 | 9 | 22 | ||
| Total | 30 | 20 | 50 | ||
Table 6. Showing the time of giving surfactant.
| Complication of the procedure | No. | % |
|---|---|---|
| Tachycardia | 18 | 36 |
| Bradycardia | 14 | 28 |
| Desaturation | 13 | 26 |
Table 7. Complication of surfactant giving procedure.
Discussion
The ideal respiratory treatment for premature newborns is still a point of contention. Because of the negative impacts of mechanical ventilation, there is a rising desire to avoid using it [21,22]. INSURE method is less invasive and have promising success rate.
This study shows INSURE success occurred in 31 neonates among 50 (62%), while failure occurred in 19 neonates (38%), which is approximately close to AL-Badry et al. (22) study that showed of the 105 neonates, successful in 71 (67.6%) while failure occurs in 34 neonates (32.4%) Cherif et al. study showed INSURE failure was registered in 35 infants (32.1%) and success in 74 infants (67.9%) this small difference may be attributed to different sample size, different criteria of INSURE failure or the use of pre-intubation medication, Morphine is a long-acting drug may increase the risk of INSURE failure and the need for mechanical ventilation [23].
Other study, Enezi (sample size 109 patient), show successful rate 101 (93%) and failure in 8 (7%) [24]. Early administration of surfactant may play an important role in the improved the prognosis of premature neonates with surfactant replacement therapy. In table no. Early administration of surfactant (<1 hour) shows significant improvement in success rate (P value 0.015).
In this study, the INSURE failure group had lower gestational age and birth weight, which is the same result in Cherif et al. while in Adel et al. study showed the same result concerning birth weight but not the gestational age due to different gestational age groups enrolled within these studies (24-34 weeks ’gestation). In Enezi study shows the same result for birth weight but high successful rate in severe prematurity group. Again, very early use of surfactant replacement therapy may be the cause in addition to good ICU and nursing care, also different sample size. Gestational age below 28 weeks required elective intubation and mechanical ventilation, that situation is not always available in our center because of limited capacity of PICU, this issue plus the definition of abortion according to Iraqi MOH is still below 28 gestational weeks, all of these issues make us exclude very low premature babies.
Concerning the Apgar score, it was show lowering in the INSURE failure group, which is the same result in Adel et al. and Cherif et al. this finding is logic as 5-minute Apgar score of 0-3 correlates with neonatal mortality in large populations [25]. Similar to this study, AL-Badry et al. showed that severe RDS was an important predictor of CPAP failure. But this is different from what was found by Cherif et al. which may be due to different study sample, difference in percentage of severe cases and different criteria for INSURE failure.
Tachycardia is most common complication during surfactant replacement therapy (36%), bradycardia (28%) and desaturation (26%).
The mortality rate in this study was 38% while Adel et al. and Cherif et al. was 30.5% (32.1%) respectively. This small difference may be due to different sample size (105 vs. 50) and (70 vs. 50) cases respectively. While in Enezi study was 7% only because of prophylactic surfactant in low prematurity, good ICU care, good nursing care and difference may be due to different sample size (109 vs. 50) and inclusion criteria.
The variables associated with failure of INSURE method were
• Less weight at birth.
• The gestational age is lower.
• Increase proportionally with severity of RDS.
• Low Apgar score at 5 minute.
Conclusion
INSURE method is good method in management of respiratory distress syndromethat associated with decreased need for mechanical ventilation and decreased neonatal mortality rate. This study shows that preterm neonates with lower birth weight, lower gestational age, lower Apgar score at 5 minutes, with severe RDS and with prolonged administrations have increased risk for INSURE method failure. Tachycardia, bradycardia and desaturation are the main complications during surfactant giving. And enough ICU beds with good nursing care appropriate with number improving outcomes of preterm patient. Every patient was received surfactant echocardiogram must be done to him for risk of PDA.
References
- Lawn JE, Blencowe H, Oza S, et al. Every Newborn: Progress, priorities, and potential beyond survival. Lancet 2014; 384(9938): 189-205.
- Wambach JA, Hamvas A. Respiratory distress syndrome in the neonate. Fanaroff and martin's neonatal-perinatal medicine. 10th edn, PA: Elsevier Saunders, Philadelphia 2015.
- Kumar A, Bhat BV. Epidemiology of respiratory distress of newborns. Indian J Pediatr 1996; 63(1): 93-8.
- Fehlmann E, José LT, Rocío F, et al. Impact of respiratory distress syndrome in very low birth weight infants: A multicenter South-American study. Arch Argent Pediatr 2010; 108(5): 393-400.
- Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet 2015; 385(9966): 430-40.
- Carlton DP, Albertine KH, Cho SC, et al. Role of neutrophils in lung vascular injury and edema after premature birth in lambs. J Appl Physiol (1985) 1997; 83(4): 1307-17.
- Nitta K, Kobayashi T. Impairment of surfactant activity and ventilation by proteins in lung edema fluid. Respir Physiol 1994; 95(1): 43-51.
- Turunen R, Nupponen I, Siitonen S, et al. Onset of mechanical ventilation is associated with rapid activation of circulating phagocytes in preterm infants. Pediatrics 2006; 117(2): 448-54.
- Naik AS, Kallapur SG, Bachurski CJ, et al. Effects of ventilation with different positive end-expiratory pressures on cytokine expression in the preterm lamb lung. Am J Respir Crit Care Med 2001; 164(3): 494-8.
- Jobe AH, Hillman N, Polglase G, et al. Injury and inflammation from resuscitation of the preterm infant. Neonatology 2008; 94(3): 190-6.
- Condò V, Cipriani S, Colnaghi M, et al. Neonatal respiratory distress syndrome: are risk factors the same in preterm and term infants?. J Matern Fetal Neonatal Med 2017; 30(11): 1267-72.
- Agrons GA, Courtney SE, Stocker JT, et al. Lung disease in premature neonates: radiologic–pathologic correlation. Radiographics 2005; 25(4): 1047-73.
- Morris SJ. Radiology of the chest in neonates. Current Paediatrics 2003; 13(6): 460-8.
- Silverman WA, Andersen DH. A controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate and necropsy findings among premature infants. Pediatrics 1956; 17(1): 1-10.
- Wood DW, Downes JJ, Lecks HI. A clinical scoring system for the diagnosis of respiratory failure: preliminary report on childhood status asthmaticus. Am J Dis Child 1972; 123(3): 227-8.
- Downes JJ, Vidyasagar D, Boggs TR, et al. Respiratory distress syndrome of newborn infants: I. New clinical scoring system (RDS score) with acid-base and blood-gas correlations. Clin Pediatr 1970; 9(6): 325-31.
- Gregory GA, Kitterman JA, Phibbs RH, et al (1971). Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. N Engl J Med 1971; 284(24): 1333-40.
- Morley CJ, Davis PG, Doyle LW, et al. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med 2008; 358(7): 700-8.
- Thukral A, Sankar MJ, Chandrasekaran A, et al. Efficacy and safety of CPAP in low-and middle-income countries. J Perinatol 2016; 36(Suppl 1): S21-8.
- Polin RA, Carlo WA, Committee on fetus and newborn, et al. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics 2014; 133(1): 156-63.
- Miller JD, Carlo WA. Pulmonary complications of mechanical ventilation in neonates. Clin Perinatal 2008; 35(1): 273-81.
- Biarent D. New tools in ventilatory support: High frequency ventilation, nitric oxide,tracheal gas insufflations, non-invasive ventilation. Pediatr Pulmonol Suppl 1999; 18: 178-81.
- AL-Badry A, Hamzah RMA, Hussein M, et al. Surfactant therapy using INSURE method (Intubation-Surfactant-Extubation) in management of respiratory distress syndrome and risk factors contributing to failure of this method. Al-Qadisiyah Medical Journal 2018; 14(1): 50-62.
- Cherif A, Hachani C, Khrouf N. Risk factors of the failure of surfactant treatment by transient intubation during nasal continuous positive airway pressure in preterm infants. Am J Perinatal 2008; 25(10): 647-52.
- Enezi FA, Mohan S, Alghamdi KF, et al. Incidence and outcome of surfactant therapy in premature neonates in ICU of KAMC. Int J Curr Microbiol App Sci 2018; 7(4): 1548-58.