Research Article - Biomedical Research (2017) Volume 28, Issue 2
Effect of specialized nursing intervention on the prognosis of hepatocellular carcinoma patients treated with sorafenib
Xufeng Pang, Guizhi Li, Yaqing Lv, Juanjuan Wang and Lili Wei*Department of Hepatobiliary and Pancreatic Surgery, Affiliated Hospital of Qingdao University, China
- *Corresponding Author:
- Lili Wei
Department of Hepatobiliary and Pancreatic Surgery
Affiliated Hospital of Qingdao University
PR China
Accepted on June 30, 2016
Abstract
Objective: A retrospective study was performed to explore the survival impact of specialized nursing care on hepatocellular carcinoma (HCC) patients receiving sorafenib therapy.
Methods: Patients with and without nursing interventions were divided into two groups. The difference in baseline characteristics and overall survival (OS) were compared between groups and prognostic factors were explored. The specialized nursing interventions consisted of education regarding selfmonitoring and adverse events management.
Results: Out of 89 patients enrolled in this study, 46 patients (51.7%, Group 1) received nursing care and 43 patients (48.3%, Group 2) did not according to the treating departments, hepatobiliary surgery and radiologic intervention, respectively. The median OS of all patients was 21.0 months (95% CI=13.7-28.3). Multivariate analysis revealed that patients without nursing care (RR=2.374, P=0.032) and pre-treatment ECOG performance score (ECOG PS) 2 (RR=6.495, P=0.001) were independent significant predictors for worse prognosis in HCC patients on sorafenib therapy. In patients with ECOG PS less than 2, the median OS of patients in Group 1 was higher than those in group 2 (38.1 months vs. 16.1 months, P=0.01).
Conclusion: Patients with pre-treatment ECOG PS 2 and those without specialized nursing interventions are independent risk factors for poor survival outcomes of advanced HCC patients receiving sorafenib therapy. Specialized nursing care may improve the prognosis of HCC patients, especially those with better ECOG PS.
Keywords
Hepatocellular carcinoma, Sarafenib, ECOG PS, Nursing care.
Introduction
Primary liver cancer is one of the common malignant diseases in mainland China, especially in coastal area. It is a highly virulent malignancy often diagnosed at late stage with a resectable rate of less than 30% [1,2]. Sorafenib, a multikinase inhibitor with anti-proliferative, anti-angiogenic and pro-apoptotic properties, was approved for treatment of unresectable Hepatocellular Carcinoma (HCC) and advanced Renal Cell Carcinoma (RCC) [3-6]. However, the incidence rate of adverse events is high with the use of sorafenib and a dose reduction or temporary interruption is required for serious adverse events. It is suggested that early recognition and management of adverse events with adequate patient education and supportive measures could minimize the chance of dose reduction and interruption of sorafenib [7,8]. Based on this, we performed a retrospective analysis of two groups of HCC patients treated with sorafenib and explore the differences in adverse reaction experience and nursing care model.
The retrospective study aimed to explore the impact of the specialized nursing intervention on patients’ prognosis.
Materials and Methods
Patient selection
Patients with radiological confirmation plus serum alpha-fetoprotein (AFP) of >400 μg/L, or histologically confirmed HCC were eligible for this study. Other inclusion criteria included Eastern Cooperative Oncology Group performance scores (ECOG PS) of 0 to 2, Child-Pugh class A or B, and patients who have received sorafenib for more than three months. Exclusion criteria included patients with concurrent heart, brain and kidney diseases, and those who received sorafenib for less than 3 months or experienced serious adverse events which resulted in treatment discontinuation.
Patient demographics and grouping
A total of 89 primary HCC patients who received oral sorafenib therapy were identified from July 2008 to August 2014. There were 83 male patients and 6 female patients, with a mean age of 54.7 years (range=37-73 years). Among them, 84 patients were hepatitis B virus carriers and 1 patient was hepatitis C virus carrier, and only 4 of them had serum marker-negative hepatitis.
All patients are divided into two groups according to the treating departments. Group 1 refers to hepatobiliary surgery outpatients followed by two nurses for specialized nursing intervention whereas Group 2 is a control group of radiology outpatients without nursing follow-up.
Sorafenib treatment and nursing interventions
All HCC patients with advanced or recurrent disease after surgery were given oral sorafenib 400 mg twice daily. Patients who experienced grade 3 or above adverse drug reaction were managed by temporary reduction to half-dose or interruption until the adverse events were resolved to at least grade 2 and the treatment was resumed to original dose.
Specialized nursing intervention included pre-treatment education of self-monitoring for drug efficacy and possible adverse drug reaction, management of adverse events during treatment, dose adjustments for serious adverse reactions, and dietary advice.
Follow-up
All patients were followed up once every four weeks which included laboratory tests of liver and renal function and serum AFP level, and radiological imaging such as ultrasound or computed tomography scan, magnetic resonance imaging, bone scan, positron emission tomography-computed tomography scan. Overall Survival (OS) was determined from the start of sorafenib therapy. Survival follow-up was censored at the time of death or on 30 November 2014. The median follow-up duration was 14.7 months (range=3.2-72.8 months).
Statistical analysis
All data were analysed using SPSS version 13.0 (SPSS, Inc., Chicago, IL). OS was estimated using Kaplan-Meier method and compared between groups using log-rank test. Multivariate analysis was performed using Cox-regression model for survival data. A P value of less than 0.05 was regarded as statistically significant.
Results
Comparison of baseline characteristics between groups
Baseline characteristics of HCC patients who received for more than 3 months of sorafenib were compared and displayed in Table 1.
| Factors | Group 1 (n=46) N (%) | Group 2 (n=43) N (%) | χ2 | P-value | |
|---|---|---|---|---|---|
| Gender | Male | 44 (95.7) | 39 (90.7) | 0.868 | 0.352 |
| Female | 2 (4.3) | 4 (9.3) | |||
| Age | ≤ 65 years | 2 (4.3) | 6 (14.0) | 2.507 | 0.113 |
| >65 years | 44 (95.7) | 37 (86.0) | |||
| HBV infection | Yes | 43 (93.5) | 41 (95.3) | 1.949 | 0.377 |
| No | 3 (6.5) | 2 (4.7) | |||
| Portal hypertension | Yes | 7 (15.2) | 7 (16.3) | 0.019 | 0.891 |
| No | 39 (84.8) | 36 (83.7) | |||
| ECOG PS | 2 | 8 (17.4) | 4 (9.3) | 1.274 | 0.264 |
| 0 or 1 | 38 (82.6) | 39 (90.7) | |||
| Child-Pugh | Class A | 44 (95.7) | 38 (88.4) | 1.625 | 0.202 |
| Class B | 2 (4.3) | 5 (11.6) | |||
| Serum AFP | >400 μg/L | 16 (34.8) | 23 (53.5) | 3.159 | 0.076 |
| ≤ 400 μg/L | 30 (65.2) | 20 (46.5) | |||
| TNM Staging | IV | 24 (52.2) | 17 (39.5) | 1.429 | 0.232 |
| ≤ III | 22 (47.8) | 26 (60.5) | |||
| Prior hepatic surgery | Yes | 34 (73.9) | 15 (34.9) | 13.682 | <0.001* |
| No | 12 (26.1) | 28 (65.1) | |||
| Hand-foot skin reaction | ≥ Grade 2 | 31 (67.4) | 24 (55.8) | 1.262 | 0.261 |
| Grade 1 | 15 (32.6) | 19 (44.2) | |||
| Diarrhoea | ≥ Grade 2 | 26 (56.5) | 12 (27.9) | 7.438 | 0.006* |
| Grade 1 | 20 (43.5) | 31 (72.1) | |||
| Dose reduction due to adverse events | Yes | 14 (30.4) | 10 (23.3) | 0.582 | 0.446 |
| No | 30 (69.6) | 33 (76.7) | |||
| Abbreviations: AFP: Alpha-Fetoprotein; ECOG PS: ECOG Performance Score; HBV: Hepatitis B Virus *Statistically significant |
|||||
Table 1. Comparison of baseline characteristics of primary liver cancer patients receiving sorafenib between groups.
When compared to Group 2 (n=43), a significantly higher percentage of patients from Group 1 (n=46) received previous hepatic surgery including liver transplantation, hepatic resection and radiofrequency ablation therapy (73.9% vs. 34.9%, P<0.001) and more patients experienced diarrhoea during sorafenib therapy (56.5% vs. 27.9%, P=0.006). Other factors were statistically insignificant between groups.
Factors associated with survival outcome
The median OS survival time was 21.0 months (95% CI, 13.7-28.3 months), and the 1-year, 2-year and 3-year survival rates were 87.2%, 75.3% and 35.2% respectively. Estimates of cumulative OS by Kaplan-Meier analysis and compared using log-rank tests showed that ECOG PS 2, Child-Pugh class B, serum AFP level of more than 400 μg/L, absence of prior hepatic surgery and absence of specialized nursing interventions were significantly associated with poor survival outcomes of primary HCC patients receiving sorafenib as shown in Table 2. Further Cox-regression analysis demonstrated that ECOG PS 2 (HR=6.495, 95% CI=2.249-18.756; P=0.001) and patients without specialized nursing interventions (HR=2.374, 95% CI=1.078-5.229; P=0.032) were independent risk factor of survival among these patients as shown in Table 2.
| Factors | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Median OS (months) | P-value | RR (95% CI) | P-value | ||
| Gender | Male | 21 | 0.726 | 0.624 | 0.596 |
| Female | 17.7 | (0.109-3.573) | |||
| Age | ≤ 65 years | 21 | 0.427 | 1.087 | 0.904 |
| >65 years | 6.7 | (0.282-4.198) | |||
| Viral Hepatitis | Negative | 24 | 0.684 | 1.513 | 0.598 |
| HBV | 22.6 | (0.324-7.060) | |||
| HCV | 12 | ||||
| Portal hypertension | No | 24 | 0.193 | 0.849 | 0.754 |
| Yes | 16 | (0.304-2.369) | |||
| ECOG PS | <2 | 28 | <0.001* | 6.495 | 0.001* |
| 2 | 7.7 | (2.249-18.756) | |||
| Child-Pugh | Class A | 24 | 0.002* | 1.46 | 0.598 |
| Class B | 6.7 | (0.435-4.901) | |||
| Serum AFP | ≤ 400 μg/L | 29.2 | 0.002* | 1.619 | 0.213 |
| >400 μg/L | 12.5 | (0.758-3.458) | |||
| TNM staging | Stage ≤ III | 24 | 0.434 | 1.304 | 0.453 |
| Stage IV | 20 | (0.652-2.609) | |||
| Prior hepatic surgery | No | 12.5 | 0.005* | 0.722 | 0.431 |
| Yes | 29.2 | (0.321-1.623) | |||
| Hand-foot skin reaction | Grade 1 | 16.1 | 0.104 | 0.788 | 0.503 |
| ≥ Grade 2 | 28 | (0.392-1.585) | |||
| Diarrhoea | Grade 1 | 16 | 0.123 | 1.071 | 0.862 |
| ≥ Grade 2 | 29.2 | (0.495-2.319) | |||
| Dose reduction due to adverse events | No | 18.2 | 0.449 | 1.296 | 0.589 |
| Yes | 25.1 | (0.506-3.318) | |||
| Specialized nursing intervention | Yes | 29.2 | 0.017* | 2.374 | 0.032* |
| No | 12.9 | (1.078-5.229) | |||
| Abbreviations: ECOG PS: ECOG Performance Score; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; OS: Overall Survival; HR: Hazard Ratio *Statistically significant |
|||||
Table 2. Univariate and multivariate survival analyses of primary liver cancer patients receiving sorafenib.
Subgroup analyses
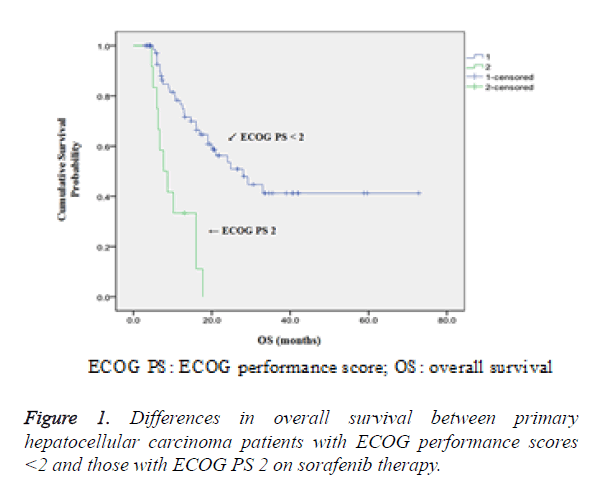
Comparison of OS between patients with ECOG PS less than 2 and those with ECOG PS 2 had shown that higher 1-year, 2- year and 3 year survival rates were observed in patients with ECOG PS<2 (86.3% vs. 11.1%, 71.7% vs. 0% and 11.1% vs. 0% respectively, P<0.05) as shown in Figure 1.
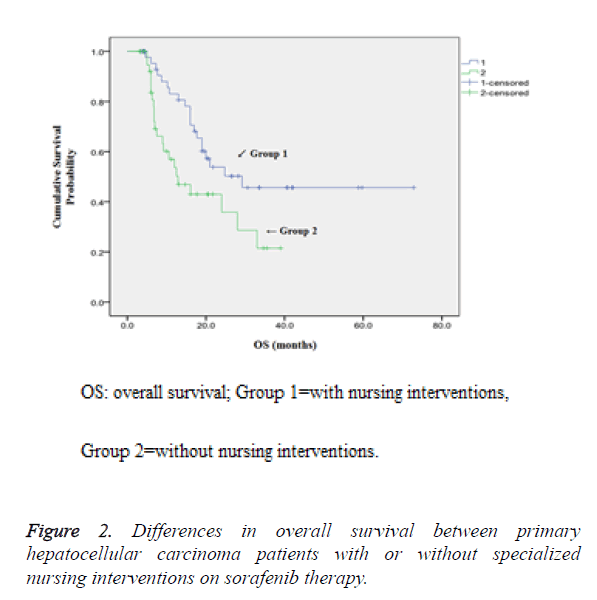
Comparison of OS between patients with or without specialized nursing interventions also demonstrated that higher 1-year, 2-year and 3-year survival rates were observed in patients who were given nursing intervention (83.1% vs. 53.6%, 57.2% vs. 35.8% and 45.7% vs. 21.5% respectively, P<0.05) as shown in Figure 2.
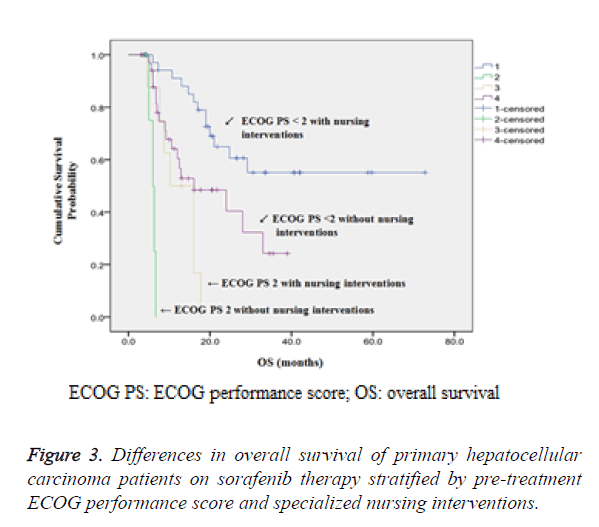
Further stratified analysis according to pre-treatment ECOG PS and patients with or without the nursing intervention has demonstrated that patients with ECOG PS of less than 2 who received nursing interventions have significantly better survival prognosis (P<0.001) as shown in Table 3 and Figure 3.
| n | Survival rate (%) | Median OS (months) | |||
|---|---|---|---|---|---|
| 1-year | 2-year | 3-year | |||
| ECOG PS <2 with nursing intervention | 38 | 91.1 | 60.6 | 55.1 | 38.1 |
| ECOG PS 2 without nursing intervention | 4 | 0 | 0 | 0 | 6 |
| ECOG PS 2 with nursing intervention | 8 | 50 | 0 | 0 | 10.2 |
| ECOG PS <2 without nursing intervention | 39 | 60.4 | 40.4 | 24.2 | 16.1 |
| Abbreviations: ECOG PS: ECOG Performance Scores; OS: Overall Survival | |||||
Table 3. Comparison of median overall survival time in patients on sorafenib therapy according to pre-treatment ECOG PS and nursing interventions.
Among 12 patients with ECOG PS 2, patients with nursing interventions had a significantly longer median OS than those without nursing interventions (10.2 months vs. 6.0 months, P=0.006). Among 77 patients with ECOG PS less than 2, the median OS was doubled for patients with nursing interventions compared to those without nursing interventions (38.1 months vs. 16.1 months, P=0.01).
Discussion
Currently, HCC treatment mainly involves surgical resection, radiofrequency ablation therapy and Transarterial Chemoembolization (TACE) depending on liver function and tumour burden of patients [9-11], but a number of patients still suffer from disease progression after treatment. The prognosis of eastern patients is even worse than patients from western counterparts such as North America and Europe [12-14].
Therefore, there is a definite unmet need for better treatment of HCC. Sorafenib, a multikinase inhibitor which inhibits Raf-1 or B-Raf kinase and targets vascular endothelial growth factor receptor-2/-3 (VEGFR-2/-3) and platelet derived growth factor receptor-beta (PDGFR-β) to block tumour cell proliferation and angiogenesis [4,15,16], was the first molecular targeted therapy to conclusively demonstrate significant improvement in OS of advanced HCC patients [3,5] and becomes the standard first-line systemic treatment [17]. In our study, we demonstrated a median OS time of 21.0 months (95% CI=13.7-28.3 months) in advanced HCC patients treated with sorafenib whereas the median OS was about 10.7 months in advanced HCC patients without prior systemic treatment from the SHAPR trial [3,18].
In our study, it is observed that pre-treatment ECOG PS of less than 2 and nursing intervention were independent predictors of good prognosis in advanced HCC patients treated with sorafenib. Eastern Cooperative Oncology Group (ECOG) developed a performance Score (PS), namely ECOG PS, is a simple measurement for quantifying patients’ general well-being and activities of daily life to assess their tolerance to cancer treatment [19]. ECOG PS, which runs from 0-5 with 0 denoting perfect performance status and 5 as death, is mainly used for assessment of performance status before chemotherapy, and patients with PS of 3 or above are not recommended for chemotherapy [19]. Luo et al. [20] validated the use of EORTC QLQ-C15-PAL questionnaire in terminal cancer patients and confirmed that patients with higher ECOG PS had more severe symptoms and worse quality of life. Sorafenib is a systemic treatment which is also associated with patients’ physical conditions. Our study excluded patients with ECOG PS ≥ 3 and demonstrated that a patient with the PS 2 is an independent predictive factor of poor prognosis.
Patients given sorafenib therapy are allowed to be treated at home, but the treatment is usually delayed for drug-related toxicities. Several studies have demonstrated that skin toxicity is a surrogate marker of sorafenib efficacy [21-23]. Shomura et al. [23] studied the relationship between adverse events and nursing intervention in 37 patients with advanced HCC who received sorafenib therapy. The study concluded that patients with skin toxicity achieved better disease control and longer OS and nursing intervention might be a good supporting measure to improve the efficacy of sorafenib. In our study, we compared patients who received nursing interventions and those who did not from different treatment departments. The result showed that patients with nursing interventions and pre-treatment ECOG PS less than 2 were independent predictive factors of good prognosis. In addition, among patients with pre-treatment ECOG PS 2, those who received nursing interventions had significantly longer median OS than those who did not (38.1 months vs. 16.1 months, P=0.01). The nursing interventions included face-to-face or telephone consultation with patients to understand their general physical conditions and inform them of medication precaution, possible adverse drug reactions and countermeasures, and the importance of adherence to treatment. Since most of the drug-induced adverse events occurred within a month of dosing, a follow-up visit was arranged every one to two weeks in the first month and subsequent follow-ups were arranged once every month.
During the nursing intervention, we noticed that patients who received surgical treatment should start sorafenib after wound healing. In our study, one patient had fat liquefaction at the incision site after hepatic resection and redness and swelling were observed at the incision site during sorafenib treatment. After stopping sorafenib for 5 days, the symptoms subsided and wound was closed and healed with two sutures. In HCC patients treated with TACE, those who experienced fever and skin flushing due to tumour necrosis after TACE should delay sorafenib therapy until the skin recovers and returns to normal. We also suggested that patients’ quality of life should be taken into consideration in addition to treatment efficacy. It sounds reasonable for dose reduction when patients experience grade 3 or above adverse events and dose interruption when necessary. From our study, temporary dose reduction or discontinuation was not correlated with disease prognosis.
Our study is however limited by the following three areas. First, our patients were recruited from two treatment out-patient departments and grouping was not performed by random assignment. Therefore, selection bias may exist in our study population. Second, the staging of disease was based on AJCC TNM classification in this study, but other prognostic factors such as portal vein invasion should also be taken into account in addition to the presence of distant metastasis. Nevertheless, we observed that the incidence of portal vein invasion was not statistically different between Group 1 and Group 2 patients (13.0% vs. 25.6%, P=0.133). Group 1 and 2 patients with portal vein invasion were further compared for median OS and again no statistical significance was observed, albeit a longer median OS was noted in Group 1 patients (10.2 months vs. 6.0 months, P=0.412), which deserves further investigation. Third, since this is a single-centre retrospective study with small sample size, further studies are required to unveil the true benefit of nursing interventions.
Conclusion
In conclusion, this preliminary study suggested that specialized nursing interventions could improve the prognosis of HCC patients treated with sorafenib therapy even though for patients with pre-treatment ECOG PS less than 2. It is therefore important to provide adequate nursing care to HCC patients during sorafenib therapy.
References
- Hung H. Treatment modalities for hepatocellular carcinoma. Curr Cancer Drug Targets 2005; 5: 131-138.
- Lovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol 2005; 40: 225-235.
- Lovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378-390.
- Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 2006; 5: 835-844.
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J,Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25-34.
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M,Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007; 356: 125-134.
- Porta C, Paglino C, Imarisio I, Bonomi L. Uncovering Pandoras vase: the growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sunitinib. Clin Exp Med 2007; 7: 127-134.
- McLellan B, Kerr H. Cutaneous toxicities of the multikinase inhibitors sorafenib and sunitinib. Dermatol Ther 2011; 24: 396-400.
- Song P. Standardizing management of hepatocellular carcinoma in China: devising evidence-based clinical practice guidelines. Biosci Trends 2013; 7: 250-252.
- Makuuchi M, Kokudo N. Clinical practice guidelines for hepatocellular carcinoma: the first evidence based guidelines from Japan. World J Gastroenterol 2006; 12: 828-829.
- Park JW. Practice guideline for diagnosis and treatment of hepatocellular carcinoma. Korean J Hepatol 2004; 10: 88-98.
- Lovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Bru C, Rodes J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatol 1999; 29: 62-67.
- Yeung YP, Lo CM, Liu CL, Wong BC, Fan ST, Wong J. Natural history of untreated nonsurgical hepatocellular carcinoma. Am J Gastroenterol 2005; 100: 1995-2004.
- A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatol 1998; 28: 751-755.
- Avila MA, Berasain C, Sangro B, Prieto J. New therapies for hepatocellular carcinoma. Oncogene 2006; 25: 3866-3884.
- Stock P, Monga D, Tan X, Micsenyi A, Loizos N, Monga SP. Platelet-derived growth factor receptor-alpha: a novel therapeutic target in human hepatocellular cancer. Mol Cancer Ther 2007; 6: 1932-1941.
- Easl E. Easl-Eortc clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908-943.
- Kane RC, Farrell AT, Madabushi R, Booth B, Chattopadhyay S, Sridhara R, Justice R, Pazdur R. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist 2009; 14: 95-100.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649-55.
- Luo ZQ, Wang N, Liu JH, Jiao J, Wu JL, Jia LN. The validation of the standard Chinese version of EORTC QLQ-C15-PAL in terminal cancer patients. Chin J Prac Nurs 2014; 30: 20-25.
- Vincenzi B, Santini D, Russo A, Addeo R, Giuliani F, Montella L, Rizzo S, Venditti O, Frezza AM, Caraglia M, Colucci G, Del Prete S, Tonini G. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncolog 2010; 15: 85-92.
- Otsuka T, Eguchi Y, Kawazoe S, Yanagita K, Ario K, Kitahara K, Kawasoe H, Kato H, Mizuta T. Skin toxicities and survival in advanced hepatocellular carcinoma patients treated with sorafenib. Hepatol Res 2012; 42: 879-886.
- Shomura M, Kagawa T, Shiraishi K, Hirose S, Arase Y, Koizumi J, Mine T. Skin toxicity predicts efficacy to sorafenib in patients with advanced hepatocellular carcinoma. World J Hepatol 2014; 6: 670-676.