Research Article - Journal of Agricultural Science and Botany (2021) Volume 5, Issue 12
Effect of several type storage conditions and preservative seed vigor of selected Acacia species.
Mulik Abbaker Ibrahim*1,2, Yan Rong Wang1, Mohammed Abdelmanan2, Salim Haroon Imam2, Nasrladeen Adam Ali2, Abdelrahim Omer Abdelrahim3
1State Key Laboratory of Grassland Agro-ecosystems, College of Pastoral, Agricultural Sciences and Technology, Lanzhou University, P.R. China
2Department of Forestry, College of Forest Sciences, University of Zalingei, Sudan
3Department of Range, College of Forest Sciences, University of Zalingei, Sudan
- *Corresponding Author:
- Mulik Abbaker Ibrahim
Department of Range College of Forest Sciences University of Zalingei Sudan
E-mail: mulikabaker@yahoo.com
Accepted on December 17, 2021
Abstract
The current study was to assess the influence of a different type storage conditions and preservative seed vigor and temperature storage method in retaining the viability of seeds of Acacia species had been successfully prophesying germination demand in several threat categories and seeds were limited store-controlled conditions due to early damage. Seeds of four A. species were stored at room temperature stored, cold stored, Arial and underground stored for a period of 0, 4, 8, 12, 16, 20 and 24 months. The results revealed that the degree of dormancy variation among the species, they were 81% for A nilotica, 74% for A. seyal, 15% for A. mellifera and 5% for A. senegal. Further, the studies were exhibited germination affected by storage time. Room storage temperature presented significantly declined in germ inability. Even more, the cold, aerial and underground storage conditions indicated effective method for preservation of A. seeds and maintenance of seed viability and storability for 24 months of storage. However, there were very few differences in response to break dormancy treatments before and after 24 months storage. As the storage period advances, A. seyal, A. mellifera and A. senegal there were progressively reduction in hard seed percentage from the beginning of the 4 months of cold, aerial and underground storage. The different responses may be reasonable by the reduction of the seed degradation in breaking dormancy and germination behavior into the room, cold, aerial and underground storage conditions can be utilized how these species might respond to environmental change, and also, used for the formation of artificial seed banks of A. species. Therefore, the aerial seed storage affects germination behavior of small seeded halophytes, further, aerial seed storage may protect seeds from detrimental salinity effects, insects and fungal are the major causes for quantitative and qualitative losses.
Keywords
Storage types, Acacia, viability, temperature, cold, Aerial, underground, germination, dormancy.
Introduction
Acacia species are growing natively in Savannah regions, there are more important sources recorded to be useful for timber, forage, gum, food, fuel, firewood, fiber, tannins, medicine, ornamentals, shade, shelter, domestic utensils, handicrafts, nitrogen fixing, soil stabilization, Agroforestry system, as well as environmental protection. Acacia seeds storage perhaps advantageous for good quality seed as well as can be harmful if the seed is of less quality, seeds will probably store for longer periods without deterioration if saved at low temperatures. The main objective of natural condition seed storage is to secure the support of good quality seed for planting and restoration programmers whenever requested. Several species produce seeds at long intervals, limiting from a few years to many years. To assure seed supply among the period between two good seed crops, a seed stock must be established [1]. Moisture content and temperature are particularly more important factors affecting the longevity of seeds and influencing the rate of deterioration [2]. The literature pertaining to the studies on effect of seed storage of Acacias is reviewed. There seems to be an association between plant ecology and seed storage behavior, it is evident that species that show recalcitrant seed storage behavior do not occur naturally in habitats that are deserted and savanna.
In some species, part of the seed with physical stored at room temperatures for several months or years becomes permeable [3,4]. Shields held at 20°C retain viability and remain soft for up to five years [5]. The evidence suggests that cold storage of 5°C is necessary to arrest the set of seed coat dormancy [6]. Long-term dry storage at room temperatures may promote the breaking of physical dormancy [7]. In these environments, the most of plant species indicated orthodox seed storage behavior, while a few indicated intermediate seed storage behavior [4]. The advantages of treating seeds before to storage it seems that, little to indicate the long period consequences of storing seeds for the non-dormant mean [8,9]. Seed conservation is ordinarily classified into two groups: ex situ and in situ [10]. Ex situ protection preserves the vegetal material exterior on the natural environment. Ex situ conservation way consists seed banks, in vivo banks and in vitro banks [11], ensuring its preservation in viable condition also saving it genetically completely for long term [12]. On the other hand, in situ method conserve the biological diversity when the management and preservation of species in the natural environment [13]. However, aerial 16seed storage are potentially the most important source of propagules for sexual regeneration in woody wetland plants, given that the seeds of woody wetland plants are often under-represented in the seed storage of wetland sediments [14,15] and that aerial seed storage have been demonstrated to play a critical role in sexual recruitment of many taxa of woody plants in terrestrial forests [16,17]. Therefore, the role of seed storage in maintaining floristic diversity in freshwater wetlands has been examined in a very large number of studies [18,19]. Further, the undergroundstorage in perennials might be important for seed development [20]. Besides, there is an urgent need to explore and develop a use for deep-underground space due to the predicted exhaustion of resources in the shallow depths of the Earth’s surface [21,22] , When the seeds deteriorate during storage, they loss their vigor and become more susceptible to environmental stresses during germination and finally are not able to germinate. The aging speed is affected by genetic and environmental factors such as temperature, seed’s moisture content and quality [23]. In other hand, the seed vigor reduces during maturity, harvesting and storage depending on temperature and moisture and gradually deteriorates [24]. The current paper describes and evaluates the impact of different types of storage condition and temperature for germination of selected Acacia seeds, in order to identify the longevity and storability in retaining the viability of seeds of Acacia species.
Materials and Methods
Seed collection
Seeds of Acacia seyal, A. nilotica, A. senegal and A. mellifera, were collected from Western Sudan and imported to the laboratory of the College of Pastoral Agricultural Sciences and Technology, Lanzhou University, China. During September 2016, Seeds were selected by sorting out the healthy, uninfected seeds of almost uniform size.
Room storage treatment
Selected seeds of A. seyal, A. nilotica, A. senegal and A. mellifera were stored at room, storage temperature at 20-25°C for 0, 4, 8, 12, 16, 20 and 24 months.
Cold storage treatment
The selected of Acacia seeds were stored at cool storage temperature at 4°C since 0, 4, 8, 12, 16, 20 and 24 months.
Aerial storage treatment
Seeds of A. seyal, A. nilotica, A. senegal and A. mellifera were stored at aerial storage temperature at 20-25 - 30°C for 0, 4, 8, 12, 16, 20 and 24 months.
Underground storage treatment
Seeds of A. seyal, A. nilotica, A. senegal and A. mellifera were stored at underground storage temperature at 15 - 20°C for 0, 4, 8, 12, 16, 20 and 24 months.
Influence of temperature on germination
Germination responses to temperature were examined for scarifying seeds incubated at five constant temperatures (15°C, 20°C, 25°C, 30°C and 35°C).
Seed germination test
Germination was carried out before and after the final of the storage experiment incubated at 20°C in incubator lighting for 12 hours a period. Samples of four replicates of 50 seeds were used for each treatment in the experiment and placed on top of 2 layers of filter paper in 90 mm Petri dishes. A seed was considered to have germinated when the radical extension was at least 0.5cm. Seeds were evaluated according to the ISTA Rules. The experiments were laid out in a completely randomized design. Comparisons in germination performance across different seed pre-treatment methods. Were made using one-way ANOVA in SPSS version 19.0.
Results
Effect of room, cold, aerial and underground storage on seed germination and hard seed performance
Germination percentage affected by A. species differed significantly over a time of storage. In most of A. senegal, A. mellifera and A. seyal the germination percentage improved during cold, aerial and underground storage, treatment to the advancement of storage time up to 4 and 8 months of storage, which later decreased at the end of storage time 24 months (Tables 1,3 and 4). However, in A. nilotica the germination percentage decreased within first 4 months of cold, aerial and underground storage after that developed in progress among the cool, aerial and underground storage period (Table 2).
| Species | Room store | GP% | HS% | DS% | AS% |
|---|---|---|---|---|---|
| A.nilotica | Control | 10ab | 81c | 0.5a | 8.5a |
| 4 m | 8.5bc | 86ab | 1a | 4.5a | |
| 8 m | 16.5a | 81.5a 82.5ab | 1a | 1a | |
| 12 m | 11.5c | 1a | 3a | ||
| 16 m | 12.5a | 83.5ab | 0a | 3a | |
| 20 m | 11ab | 79a | 0.5a | 0a | |
| 24 m | 5d | 72a | 2a | 1a | |
| Cool store | |||||
| 4 m | 4.5a | 89c | 0.5a | 2.5a | |
| 8 m | 14.5ab | 85bc | 0.5a | 2a | |
| 12 m | 18.5bc | 81.5ab | 0a | 0a | |
| 16 m | 22c | 78a | 0a | 2a | |
| 20 m | 20bc | 78b | 0.5a | 1.5a | |
| 24 m | 12c | 88a | 0a | 0a | |
| Underground | |||||
| 4 m | 9.5bc | 84ab | 1.5a | 5ab | |
| 8 m | 7c | 89.5a | 0a | 3.5b | |
| 12 m | 12.5a | 84.5ab | 0a | 3b | |
| 16 m | 11ab | 86.5ab | 0a | 2.5b | |
| 20 m | 12a | 83.5ab | 0.5a | 3b | |
| 24 m | 12.5ab | 80ab | 3a | 4.5b | |
| Aerial store | |||||
| 4 m | 4c | 89a | 2.5a | 4.5a | |
| 8 m | 10.5b | 87a | 1ab | 1.5ab | |
| 12 m | 14b | 83.5a | 0c | 2.5b | |
| 16 m | 14b | 85a | 0c | 1b | |
| 20 m | 30.5a | 64b | 1.5b | 4.5a | |
| 24 m | 26ab | 67.5ab | 2.5a | 4a | |
| Means within a seed sample marked by different letters are significantly different at the 5%. | |||||
Table 2. Effect of room, cold, aerial and underground storage condition on seed germination of A. nilotica during 24 months.
In contrast, the germination percentage remarkable gradual declined in the whole of A. senegal, A. mellifera, A. seyal and A. nilotica at room, storage time (Table 1and 4). Before starting storage, treatment, the seed of A. seyal and A. nilotica indicated no significant differences in seed viability. This may indicate that the of seed species were highly viable. Further, hard seed percentage as impacted by A. species differed significantly during storage time (Table 1 and 2). As the storage period advances, there were progressively reduction in hard seed percentage from the beginning of the 4 months of cold, aerial and underground storage seed of A. senegal, A. seyal and A. mellifera. As well as, the hard seed percentage of A. nilotica was increased in the first 4 months and then declined advanced during at end of cool storage (Table 1). The death seed percentage of those A. species effected by differed significantly above the period of 24 months of cold, aerial and underground storage. Since, storage time advances, the significant increasing in the dead seed percentage recorded in A. senegal and A. mellifera exhibited decline. Furthermore, the dead seed percentage was highest at the roomy storage as compared to cold, aerial and underground storage at the end of 24 months of storage time (Tables 3 and 4). The abnormal seed percentage of those A. species influenced by differed significantly during the period of 24 months of cold, aerial and underground storage. At the storage time advances there was a significant increase in the abnormal seed percentage of A. senegal and A. mellifera (Tables 3and Table 4). Even more, the abnormal seed percentage was decreased at cold, aerial and underground storage for A. senegal, A. nilotica and A. mellifera among the end of the 24 months of storage time (Tables 1-4).
| Species | Room store | GP% | HS% | DS% | AS% |
|---|---|---|---|---|---|
| A.seyal | Control | 22.5a | 74c | 1a | 2.5a |
| 4 m | 18ab | 76.5b | 1a | 4.5a | |
| 8 m | 17.5ab | 78b | 1.5a | 3a | |
| 12 m | 13.5b | 82.5ab | 1a | 3a | |
| 16 m | 13.5b | 83.5ab | 0a | 3a | |
| 20 m | 10.5c | 89a | 0.5a | 0a | |
| 24 m | 5d | 92a | 2a | 1a | |
| Cool store | |||||
| 4 m | 28.5a | 69c | 0.5a | 2.5a | |
| 8 m | 22.5ab | 75bc | 0.5a | 2a | |
| 12 m | 18.5bc | 81.5ab | 0a | 0a | |
| 16 m | 14.5c | 83.5a | 0a | 2a | |
| 20 m | 20bc | 78b | 0.5a | 1.5a | |
| 24 m | 12c | 88a | 0a | 0a | |
| Underground | |||||
| 4 m | 7.5bc | 86ab | 1.5a | 5ab | |
| 8 m | 8c | 87a | 2a | 3b | |
| 12 m | 12.5a | 84.5ab | 0a | 3b | |
| 16 m | 11ab | 87.5ab | 0a | 1.5b | |
| 20 m | 12a | 83.5ab | 0.5a | 3b | |
| 24 m | 11.5ab | 84ab | 3a | 1.5b | |
| Aerial store | |||||
| 4 m | 8c | 85a | 2.5a | 4.5a | |
| 8 m | 10.5b | 87a | 1ab | 1.5ab | |
| 12 m | 14b | 83.5a | 0c | 2.5b | |
| 16 m | 14b | 85a | 0c | 1b | |
| 20 m | 25a | 70b | 0.5b | 4.5a | |
| 24 m | 17ab | 77.5ab | 2.5a | 3a | |
| Means within a seed sample marked by different letters are significantly different at the 5%. | |||||
Table 1. Effect of room, cold, aerial and underground storage condition on seed germination of A. seyal, during 24 months.
| Species | Room store | GP% | HS% | DS% | AS% |
|---|---|---|---|---|---|
| A.mellifera | Control | 80.5a | 15c | 2a | 2.5a |
| 4 m | 81ab | 6.5b | 5a | 4.5a | |
| 8 m | 77.5ab | 5b | 7a | 3a | |
| 12 m | 71.5b | 4.5ab | 11a | 3a | |
| 16 m | 53.5b | 4.5ab | 15a | 3a | |
| 20 m | 60.5c | 5a | 18a | 0a | |
| 24 m | 45d | 7a | 16a | 26a | |
| Cool store | |||||
| 4 m | 88.5a | 2c | 6.5a | 3a | |
| 8 m | 82.5ab | 4.5bc | 10a | 3a | |
| 12 m | 78bc | 5ab | 15a | 2a | |
| 16 m | 75c | 7a | 14a | 4a | |
| 20 m | 70bc | 8b | 15a | 7a | |
| 24 m | 63c | 10a | 17a | 10a | |
| Underground | |||||
| 4 m | 87bc | 7ab | 1a | 5ab | |
| 8 m | 88c | 6.5a | 2a | 3.5b | |
| 12 m | 92a | 5ab | 0a | 3b | |
| 16 m | 90ab | 8.5ab | 0a | 1.5b | |
| 20 m | 92a | 5.5ab | 0.5a | 2b | |
| 24 m | 90ab | 4ab | 3a | 3b | |
| Aerial store | |||||
| 4 m | 90c | 3a | 2.5a | 4.5a | |
| 8 m | 87.5b | 10a | 1ab | 1.5ab | |
| 12 m | 84b | 13.5a | 0c | 2.5b | |
| 16 m | 80b | 19a | 0c | 1b | |
| 20 m | 77a | 18b | 0.5b | 4.5a | |
| 24 m | 70ab | 24ab | 2a | 4a | |
| Means within a seed sample marked by different letters are significantly different at the 5%. | |||||
Table 3. Effect of room, cold, aerial and underground storage condition on seed germination of A. mellifera during 24 months.
| Species | Room store | GP% | HS% | DS% | AS% |
|---|---|---|---|---|---|
| A.senegal | Control | 83.5a | 13c | 1a | 2.5a |
| 4 m | 80ab | 14b | 1a | 4.5a | |
| 8 m | 78.5ab | 17b | 1.5a | 3a | |
| 12 m | 75.5b | 21ab | 1a | 3a | |
| 16 m | 70.5b | 26.5ab | 0a | 3a | |
| 20 m | 60.5c | 40a | 0.5a | 0a | |
| 24 m | 55d | 47a | 2a | 1a | |
| Cool store | |||||
| 4 m | 91a | 6c | 0.5a | 2.5a | |
| 8 m | 90a | 8bc | 0.5a | 2a | |
| 12 m | 85bc | 15ab | 0a | 0a | |
| 16 m | 84c | 14a | 0a | 2a | |
| 20 m | 81bc | 17b | 0.5a | 1.5a | |
| 24 m | 82c | 18a | 0a | 0a | |
| Underground | |||||
| 4 m | 85ab | 9ab | 1a | 5ab | |
| 8 m | 84c | 12.5a | 0a | 3.5b | |
| 12 m | 90a | 7b | 0a | 3b | |
| 16 m | 85ab | 13.5a | 0a | 1.5b | |
| 20 m | 88a | 9ab | 0.5a | 3b | |
| 24 m | 85ab | 11ab | 3a | 1b | |
| Aerial store | |||||
| 4 m | 93a | 6b | 0.5a | 0.5b | |
| 8 m | 90.5a | 7b | 1a | 1.5b | |
| 12 m | 84ab | 6b | 5a | 5b | |
| 16 m | 84ab | 6b | 5a | 5b | |
| 20 m | 80b | 10ab | 5.5a | 4.5b | |
| 24 m | 75c | 12a | 5a | 8a | |
| Means within a seed sample marked by different letters are significantly different at the 5%. | |||||
Table 4. Effect of room, cold, aerial and underground storage condition on seed germination of A. senegal during 24 months.
Impact of temperature on seed germination performance
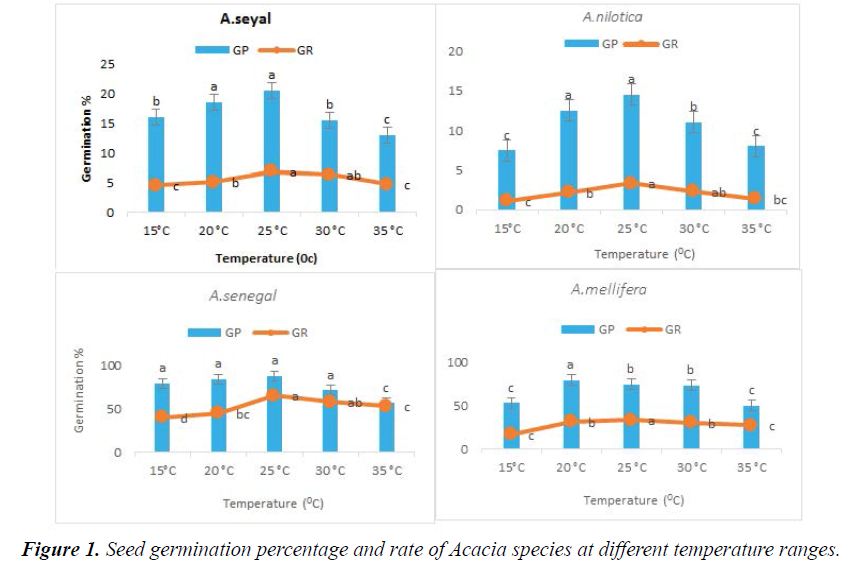
The temperature had significant effects on the percentage and germination rate (1/T50) of each species (P < 0.05). Germination percentage of A. mellifera and A. senegal permeable seed dormancy was resulted in high germination at 20°C, 75%, 84.5 % and 25°C, 80%, 88.5%, respectively. Moreover, significantly decreased by 30°C 15.5%, 11% and 35°C, 13%, 8%. Germination percentages of A. seyal and A. nilotica impermeable seed dormancy were shown germination at 20°C, 18.5%, 12.5 % and 25°C, 20.5%, 14.5%, respectively. Further, significantly diminished by 30°C and 35°C. Germination rates generally improved as temperature increased and then decreased as temperature increased. Germination rate usually improving linearly with increased temperature up to an optimum point, after that, diminished linearly to a ceiling temperature (Figure 1).
germination under the different condition’s storage tested, and germination after 24 months storage. The recorded, evidently performance the existence of room, cold, aerial and underground storage condition correlated variation in the seed germination behavior of those A. species and a significant influence of period of room, cold, aerial and underground storage on germination. Dormancy has broken within the room; cold, aerial and underground storage exposed two main groups of species, indicating contrasting germination behavior in decreased or increased germ inability. In the whole two comparisons depicted the storage condition may strongly influence seed germination. The distribution of seed germination percentages revealed that the cold, aerial and underground storage is the more effective condition for dormancy broken compared to the room storage condition duration of seed storage. Seeds by deep and no deep physical dormancy was stored below room, cold, aerial and underground store conditions, timing of sowing, there were three stages to consider. Firstly, dormancy loss can exist room and cold storage, but it can not to be complete, some of the seeds can change non-dormant and remain dormant. Secondly, the rate of dormancy broken becomes different between the species. Thirdly, several seeds can lose viability within the room and cool storage, also again we had shown that can vary among the species and collection area. Furthermore, the moisture content of the seeds is one of the most important factors determining seed longevity during storage [25,26,1]. Seed survival during the period of longevity varies greatly within species. It can also vary between accessions among a species due to differences in genotype and location. Affected by the area in potential longevity recorded from the environment during seed harvesting, maturation, duration of drying, time of seed harvest, pre-storage environment and the period of seed storage [27- 30]. Besides, studies about the long-term viability are requested for the storability of those Acacia seeds in the gene bank. The different storage conditions response may have affected by many factors, including seeds structure, intrinsic physiological characters of each species and environmental factors such as temperature, moisture content and humidity [31,32]. Therefore, the temperature responses of the plants to view the environments which they are adaptable. Even more, differences within the species have substantial ecological significance [33]. Species from tropical regions ordinarily require high-level temperature for germination in temperate species. The optimal temperature for germination of four Banksia species has been positively correlated together with mean annual temperature in the area of seed originates [34]. For the current study the seed germination of Acacia species completely more tolerant of low temperature. In conclusion, the cold, aerial and underground conditions of seed storage for A. nilotica, A. senegal, A. mellifera and A. seyal remained completely viable among 24 months of storage. However, the room, storage condition gradually declined seed viability during 24 months of storage and did not maintain seed viability. The variation within cold, room, aerial and underground storage may be due to differences in the storage conditions, temperature and moisture content. There were very few differences in response to break dormancy treatments before and after 24 months storage. According to these findings, storing seeds of a permeable seed’s dormancy of A. senegal and A. mellifera, revealed does not change germination and this fascinates significant interest to the current seed-based restoration programs and promoted seed use effectiveness. The potential of dormancy broken to find among cold, room, aerial and underground storage must be considered when examined on initial seed dormancy were conducted maybe when seeds from different locations were stored before to propagating plants. It is to suggest that fresh seeds must be used after collection before proceeding for experimentation. Although, the aerial seed storage protects seeds against granivores, help seed dispersal over time and maintain seeds in a favorable microhabitat.
References
- Wang JH, Baskin CC, Chen W, et al. Variation in seed germination between populations of five sub-alpine woody species from eastern Qinghai-Tibet Plateau following dry storage at low temperatures. Ecol Res 2010;25(1):195-203.
- Spanò C, Buselli R, Castiglione MR, et al. R Nases and nucleases in embryos and endosperms from naturally aged wheat seeds stored in different conditions. J Plant Physiol 2007;164(4):487-95.
- Govender V, Aveling TA, Kritzinger Q. The effect of traditional storage methods on germination and vigour of maize (Zea mays L.) from northern KwaZulu-Natal and southern Mozambique. S Afr J Bot 2008;74(2):190-6.
- Alamgir M, Hossain MK. Effect of pre-sowing treatments on germination and initial seedling development of Albizia saman in the nursery. J For Res 2005;16(3):200-4.
- Coaldrake JE. Variation in some floral, seed, and growth characteristics of Acacia harpophylla (brigalow). Aust J Bot 1971;19(3):335-52.
- Isikawa S, Ishikawa T. Requirement of low temperature treatment following illumination in the germination of seed of Elsholtzia. Plant Cell Physiol 1960;1(2):143-9.
- Meisert A. Physical dormancy in Geraniaceae seeds. Seed Sci Res 2002;12(2):121-8.
- Rowe HI. Tricks of the trade: techniques and opinions from 38 experts in tallgrass prairie restoration. Restoration Ecology 2010;18:253-62.
- Merritt DJ, Dixon KW. Restoration seed banks-a matter of scale. Sci 2011;332(6028):424-5.
- Draper D, Marques I, Graell AR, et al. Conservação de Recursos Genéticos–O Banco de Sementes “António Luís Belo Correia”. Curso Avançado sobre “Métodos de conservação a longo prazo de recursos fitogenéticos: conservação pelo frio. 2004:3-7.
- Assis JGA, Resende SV, Bellintani MC, et al. Conservação ex situ. Plano de Ação Nacional para a Conservação das Cactáceas-Série Espécies Ameaçadas. 2011;(24):44-54.
- Hay FR, Probert RJ. Advances in seed conservation of wild plant species: a review of recent research. Conservation physiology. 2013;1(1).
- Teixido AL, Toorop PE, Liu U, et al. Gaps in seed banking are compromising the GSPC’s Target 8 in a megadiverse country. Biodivers Conserv 2017;26(3):703-16.
- Middleton BA. Soil seed banks and the potential restoration of forested wetlands after farming. J Appl Ecol 2003;40(6):1025-34.
- Capon SJ, Brock MA. Flooding, soil seed bank dynamics and vegetation resilience of a hydrologically variable desert floodplain. Freshw Biol 2006;51(2):206-23.
- Barrett LG, He T, Lamont BB, et al. Temporal patterns of genetic variation across a 9‐year‐old aerial seed bank of the shrub Banksia hookeriana (Proteaceae). Mol Ecol 2005;14(13):4169-79.
- Russell‐Smith J, Setterfield SA. Monsoon rain forest seedling dynamics, northern Australia: contrasts with regeneration in eucalypt dominated savannas. J Biogeogr 2006;33(9):1597-614.
- Boedeltje G, Bakker JP, ter Heerdt GN. Potential role of propagule banks in the development of aquatic vegetation in backwaters along navigation canals. Aquat Bot 2003;77(1):53-69.
- Liu GH, Li W, Li EH, et al. Landscape scale variation in the seed banks of floodplain wetlands with contrasting hydrology in China. Freshw Biol 2006;51(10):1862-78.
- Amartuvshin N, Ochgerel N. Yearly Difference in Normalised Seed Weight of Cultivated Iris dichotoma Pall in Mongolia. J Agric Biotech 2016;1(01).
- Ranjith PG, Zhao J, Ju M, et al. Opportunities and challenges in deep mining: a brief review. Engineering 2017;3(4):546-51.
- Rezayian M, Niknam V, Ebrahimzadeh H. Effects of drought stress on the seedling growth, development, and metabolic activity in different cultivars of canola. Soil Sci Plant Nutr 2018;64(3):360-9.
- Ma F, Cholewa EW, Mohamed T, et al. Cracks in the palisade cuticle of soybean seed coats correlate with their permeability to water. Ann Bot 2004;94(2):213-28.
- Marshall AH, Lewis DN. Influence of seed storage conditions on seedling emergence, seedling growth and dry matter production of temperate forage grasses. Seed Sci Technol 2004;32(2):493-501.
- Qaisrani R. Safe storage of cereals at higher moisture levels. Research report, CSIRO, Stored Grain Research Laboratory, Kangaroo Island, SA; 2000.
- Baskin CC, Thompson K, Baskin JM. Mistakes in germination ecology and how to avoid them. Seed Sci Res 2006;16(3):165-8.
- Hong TD, Ellis RH. A protocol to determine seed storage behaviour. Bioversity International; 1996.
- Verdú M. Tempo, mode and phylogenetic associations of relative embryo size evolution in angiosperms. J Evol Biol 2006;19(2):625-34.
- Bu H, Chen X, Xu X, et al. Seed mass and germination in an alpine meadow on the eastern Tsinghai–Tibet plateau. Plant Ecol 2007;191(1):127-49.
- Bu H, Du G, Chen X, et al. Community-wide germination strategies in an alpine meadow on the eastern Qinghai-Tibet plateau: phylogenetic and life-history correlates. Plant Ecol 2008;195(1):87-98.
- Davies RM, Newton RJ, Hay FR, et al. 150-seed comparative longevity protocol–a reduced seed number screening method for identifying short-lived seed conservation collections. Seed Sci Technol 2016;44(3):569-84.
- Meiado MV, Rojas Aréchiga M, de Siqueira Filho JA, et al. Effects of light and temperature on seed germination of cacti of B razilian ecosystems. Plant Species Biol 2016;31(2):87-97.
- Liu W, Liu K, Zhang CH, et al. Effect of accumulated temperature on seed germination—A case study of 12 Compositae species on the eastern Qinghai-Tibet Plateau of China. Chin J Plant Ecol 2011;35(7):751.
- Cochrane A, Hoyle GL, Yates CJ, et al. Predicting the impact of increasing temperatures on seed germination among populations of Western Australian Banksia (Proteaceae). Seed Sci Res 2014;24(3):195-205.