- Biomedical Research (2014) Volume 25, Issue 1
Effect of Nigella sativa on experimental liver fibrosis.
Edibe Saricicek1, Mehmet Tarakcioglu2, Vahap Saricicek3,*, Murat Taner Gulsen4, Metin Karakok5, Yasemin Baltaci6, Seyithan Taysi21Department of Biochemistry and Clinical Biochemistry, Dr. Ersin Arslan State Hospital, Gaziantep, Turkey,
2Department of Medical Biochemistry, Gaziantep University, Medical Faculty, Gaziantep, Turkey
3Department of Anesthesiology, Gaziantep University, Medical Faculty, Gaziantep, Turkey
4Department of Gastroenterology, Gaziantep University, Medical Faculty, Gaziantep, Turkey
5Department of Pathology, Gaziantep University, Medical Faculty, Gaziantep, Turkey
6Department of Physiology, Gaziantep University, Medical Faculty, Gaziantep, Turkey
- *Corresponding Author:
- Vahap Saricicek
Gaziantep University Medical Faculty
Department of Anesthesiology and Reanimation
27310 Sahinbey, Gaziantep, TURKEY
Accepted Date: October 21, 2013
Abstract
Our objective was to investigate the effects of NS, Nigella Sativa oil (NSO) and thymoquinone (TQ) on liver fibrosis induced by dimethylnitrosamine (DMN) in rats. Our study groups consisted of five subgroups: the control, DMN, TQ, NS, and NSO groups. At the end of the study, the animals were sacrificed, and blood and tissue samples were obtained. Malondialdehyde (MDA) levels, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) activity, and routine biochemical parameters were analyzed. Histopathologic evaluation of the NS, NSO and TQ groups found normal liver tissue and short fibrosis, and fibrous expansion with or without a septum and in some portal areas. The DMN group evaluation found incomplete cirrhosis in the liver tissues of rats. While erythrocyte and tissue MDA levels were higher, SOD and GSH-Px activities were lower in the NS, NSO and TQ groups when compared to the DMN group. Our results showed that NS and its components reduced oxidative stress and had antioxidant effects and free-radical scavenging activity. NS also protected the antioxidant enzyme activities in the liver tissue of rats.
Keywords
Nigella Sativa, Nigella Sativa Oil, Thymoquinone, Liver Fibrosis
Introduction
Liver cirrhosis is a prevalent disease worldwide, especially in populations where hepatotropic virus infections (B, C and Delta) are endemic and in western societies where high alcohol consumption is common. Cirrhosis is the fourth most common cause of death in the United States (US) [1]. Fibrotic processes begins from disse’s space, and progress to pericentral area resulting in panlobular fibrosis, thus nodule formation is developed. Cirrhosis has poor prognosis unless liver transplant is carried out [2].
Hepatic fibrosis is a consequence of severe liver damage, and many chronic liver diseases often may progress to cirrhosis [3,4]. Over production of extracellular matrix (ECM) compounds through the activation of liver cells is seen in liver fibrosis [5]. Liver fibrosis or cirrhosis can be reversible in contrast to previously known [6]. For this reason, new treatment modalities have been focused on the decreases of the accumulation, or reduced the progression of fibrosis [7].
To control the flux of reactive oxygen species (ROS) in physiological conditions, aerobic cells have developed their own defense system against free radical attacks: the antioxidant system, which includes both enzymatic and non-enzymatic components. This system consists of low molecular weight antioxidant molecules and various antioxidant enzymes, including, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and glutathione-Stransferase (GST) [8,9].
Nigella sativa (NS) is a member of the Ranunculaceae family, and today it is a plant commonly grown and cultivated mainly in the east Mediterranean region and other countries [10]. NS contains 0.4-0.45% volatile oil and more than 30% constant oil; 18-24% of these volatile oils are composed of thymoquinone (TQ) [11]. NS has been proven to have bronchodilator, antibacterial, antihyperten-sive, antidiabetic, gastro-protective, antihistaminic, antioxidative, neuro-protective, and hepatoprotective effects [12]. TQ is the most significant active component of NS. Numerous studies have found that TQ is a hepatoprotective and potent antioxidant against liver damage induced by carbon tetrachloride and tert-butyl hydroperoxide agents [13].
To best of our knowledge, there is no experimental study thatthat simultaneously investigates the effects of NS, nigella sativa oil (NSO) and TQ supplementation on DMN-induced hepatotoxicity. In this study, we aimed to determine the effects of TQ, NS and NSO on DMNinduced liver fibrosis in rats.
Materials and Method
Animals and experimental protocol
This study was carried out in the Medical Biochemistry Laboratories at the Medical School of Gaziantep University. The study was approved by the local ethics committee for laboratory animals. In this study, we used 50 male Wistar-Albino rats whose weights ranged from 200-250 grams. These animals were kept at 21°C in an environment with a 12-hour light and a 12-hour dark cycle. They were fed standard rat food. Before the beginning of the study, rats with unsuitable health conditions were removed from the main population.
The rats were randomly assigned to one of five experimental groups, each consisting of ten animals. The control group was injected with intraperitoneal (ip) physiological saline; and the other groups received 1% DMN 10 mg/kg ip for three consecutive days a week for three weeks in a row. DMN treatment was terminated by the end of the third week, and the other groups’ treatment lasted for ten days, during which the groups were given NS, NSO and TQ.
▪ Control group: The rats received standard food and water.
▪ NS group: NS was added to the animals’ food.
▪ NSO group: 2.4 gr/kg/day NSO was given through oral gavage.
▪ TQ group: 50 mg/kg/day TQ was given to this group through oral gavage.
Biochemical analysis
At the end of the experiment, the animals were anesthetized with ketamine–HCl (Ketalar, 20 mg/kg−1, ip), and the blood was collected by cardiac puncture after thoracotomy. Blood samples were collected in Vacutainer tubes both without and with K3-EDTA as an anticoagulant. Then all the animals were sacrificed through decapitation to remove their livers. Half of the liver samples were spared for pathologic analysis in 10% neutral formalin solution. For determination of biochemical parameters, the liver tissue was homogenized in physiological saline solution. The homogenate was centrifuged at 10,000×g for 10 minutes to remove debris. The clear upper supernatant was collected, and all assays in the liver tissue were carried out on this fraction. This procedure was performed in a refrigerated environment.
The blood samples were centrifuged at 3,000×g for 10 min, and plasma and serum were removed using a Pasteur pipette. Then, erythrocytes were washed with 0.9% NaCl solution three times, and the washed erythrocytes were hemolyzed by dilution with deionized water (50-fold). Hemoglobin (Hb) values of the samples were measured using a Sysmex hematology analyzer. The hemolysate, serum and homogenates were kept at −80°C until biochemical determinations.
Measurement of erythrocytes and liver MDA levels
Erythrocytes and liver MDA levels were measured with the methods described by Jain et al. and Ohkawa et al., respectively [14,15]. Ohkawa method, MDA was determined by spectrophotometry of the pink-colored product of the thiobarbituric acid-reactive substances complex. Total thiobarbituric acid-reactive substances (TBARS) were expressed as MDA. Erythrocytes and liver MDA levels were expressed as nmol/mgHb and nmol/mg protein, respectively.
Measurement of SOD activity
Erythrocyte SOD activity was assessed according to the method described by Sun et al. In this method, the xantine– xantine oxidase complex produces superoxide radicals, which react with nitroblue tetrazolium (NBT) to form the formazan compound [16]. SOD activity is measured at 560 nm by detecting the inhibition of this reaction. By using a blank study in which all reagents except a supernatant sample were present and by determining the sample and blank absorbance, activity was calculated and is given below. One SOD unit was defined as the enzyme amount causing 50% inhibition in the NBTH2 reduction rate. SOD activities in the liver tissue and erythrocytes were also expressed as U/mg protein and U/mg Hb, respectively.
Measurement of GSH-Px activity
GSH-Px activity was measured by coupled spectrophotometric assay at 340 nm from the oxidation of nicotinamide adenine dinucleotide phosphate (NADPH) in the presence of hydrogen peroxide (H2O2) used as a substrate. GSH-Px activity was measured according to the method of Paglia and Valentina [17]. In this procedure, GSH-Px catalyses the oxidation of glutathione in the presence of tert-butyl hydroperoxide. Oxidized glutathione is converted to its reduced form in the presence of glutathione reductase and NADPH, while NADPH is oxidized to NADP+. The decrease in the absorbance of NADPH at 340 nm was also measured. By calculating the absorbance change per minute and by using the molar extinction coefficient of NADPH, the GSH-Px activity in the erythrocytes and liver tissue could be calculated. Erythrocytes and liver tissue GSH-Px activities were expressed as U/gr Hb and U/gr protein, respectively.
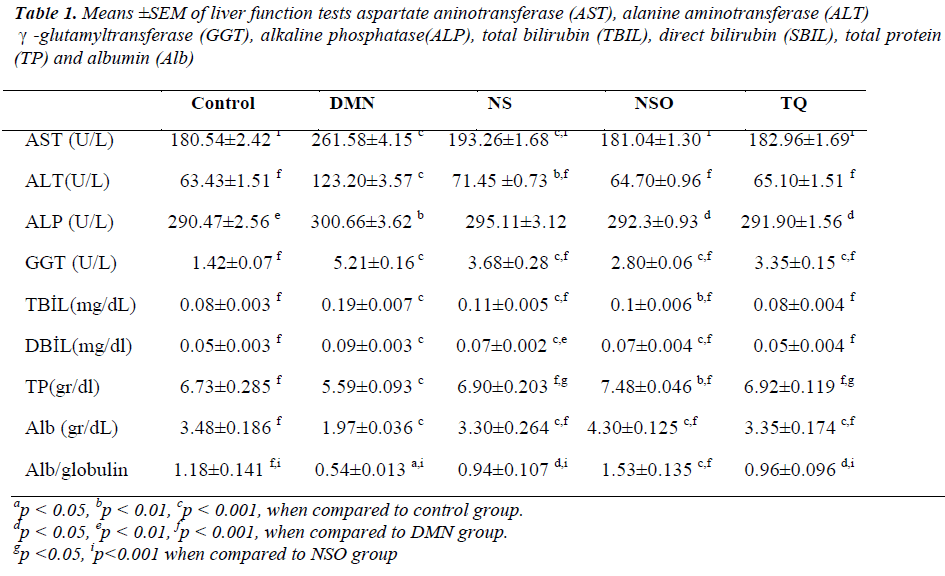
The aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), alkaline phosphatase (ALP), total bilirubin (TBIL), direct bilirubin (DBIL), total protein (TP), and albumin (Alb) levels were measured using a commercial kit (Cormay, Japan) and with a Prestige 24i appliance (Japan, Tokyo Boeki). In contrast, ALT, AST, GGT enzymaticcolorimetric, ALP, TBIL, DBIL, TP, and Alb levels were measured via the colorimetric method. Protein assignment for liver tissue was performed through the Bradford method [18].
Histopathological examination of the liver
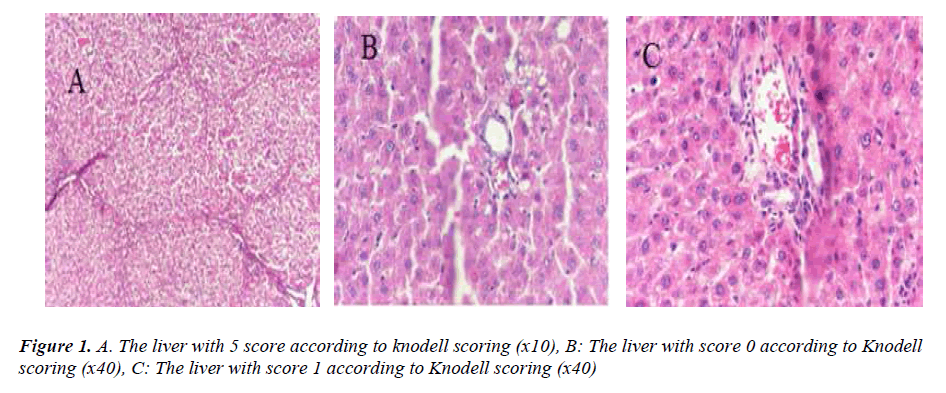
Half of the samples from the livers were examined with a photo-microscope at a 400 times magnification level after histochemical dyeing with Hematoxilen-Eosin and Masson- Tricrom paints. The preparations were evaluated zero to six, according to modified Knodell scoring [19].
Statistical analysis
All the parameters included in the study were compared using a one-way analysis of variance (ANOVA). Descriptive values for all the group parameters, including minimum and maximum means and standard errors, were calculated. The normal distribution of the data obtained was checked through the Kolmogorov Smirnov test. The results were analyzed within a 95% confidence interval and at a p<0.05 critical level. The results are expressed as mean ± SEM.
Results
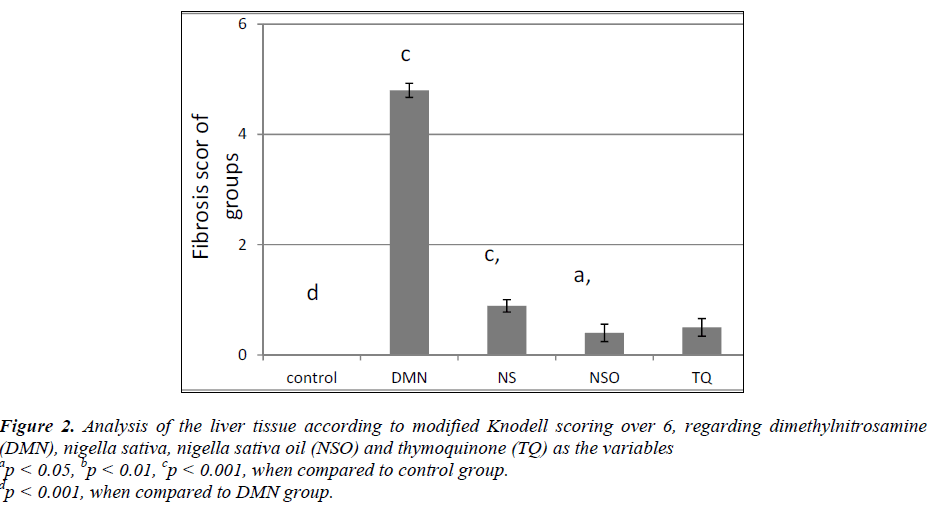
The analysis of the data made it quite clear that, in terms of Knodell scoring, the fibrosis values of the DMN group revealed a statistically significant difference (p<0.001) compared to all the other groups. Additionally, based on the histopathological appearances, formations of distinct bridging (porto-portal and/or porto-central) and incomplete cirrhosis were observed along with nodules (Fig. 1A). After hepatic fibrosis, it was determined that fibrosis values of the rats in the NS, NSO and TQ groups were significantly lower compared to the DMN group in terms of Knodell modified scoring (p<0.001) (Fig. 2). Histopathologically, short fibrosis and enlargement in fibrosis (with/without septum) were detected in the normal liver tissues and in some portal areas (Figs. 1B, C).
Figure 2: Analysis of the liver tissue according to modified Knodell scoring over 6, regarding dimethylnitrosamine (DMN), nigella sativa, nigella sativa oil (NSO) and thymoquinone (TQ) as the variables ap < 0.05, bp < 0.01, cp < 0.001, when compared to control group. dp < 0.001, when compared to DMN group.
Compared to the DMN group, statistically significant decreases were detected in ALT, AST, GGT, ALP, TBIL, and DBIL levels in the other groups (p<0.001, p<0.001, p<0.001, p<0.05, p<0.001, and p<0.001, respectively). In addition, TBIL and DBIL levels of the TQ group were found to be significantly low compared to the NSO groups (p<0.01 and p<0.001). Compared to the DMN group, the TP and Alb levels of the other groups significantly increased (p<0.001 and p<0.001). It was also determined that TP and Alb levels of the NSO group significantly increased compared to the TQ and NS groups (p<0.05). Compared to the other groups, there was a significant decrease in the albumin/globulin ratio of the DMN group (p<0.05). In the NSO group, the albumin/ globulin ratio significantly increased when compared to the other groups (p<0.001) (Table 1).
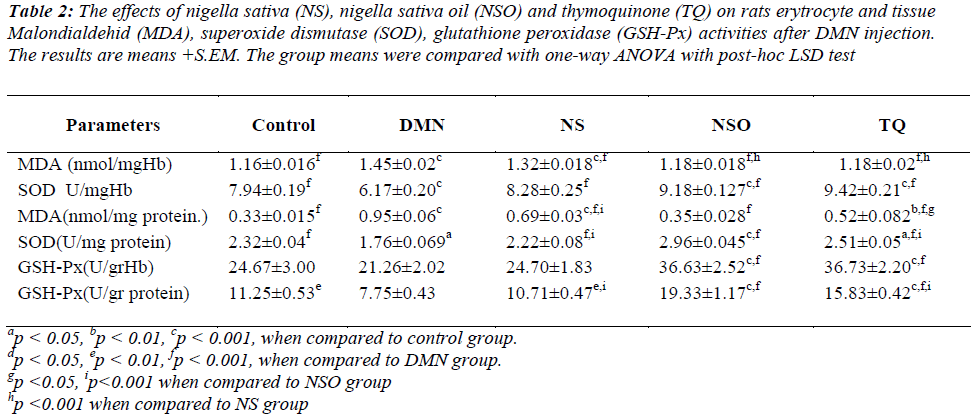
When the liver MDA levels of the DMN group were compared to the other groups, a statistically significant increase was determined (p<0.001); in addition, the liver SOD and GSH-Px activities in the tissue were also found to be significantly lower (p<0.001 and p<0.001, respectively). Furthermore, while the tissue MDA values were significantly lower in the NSO group compared to the TQ and NS groups (TQ group: p<0.05, NS group: p<0.001), tissue SOD and GSH-Px values were significantly increased (p<0.001, p<0.001).
Erythrocyte MDA levels in the DMN group were determined to be significantly high (p<0.001 for all) when compared to the other groups; in addition, erythrocyte SOD and GSH-Px activities were found to be statistically lower (p<0.001, p<0.001).
In this study, when the NSO group and TQ group were compared, there seemed to be no significant difference in erythrocyte MDA level, SOD and GSH-Px activities; however, a significant decrease in the erythrocyte MDA values in the NSO and the TQ groups was observed compared to the NS group (p<0.001, p<0.001) (Table 2).
Table 2: The effects of nigella sativa (NS), nigella sativa oil (NSO) and thymoquinone (TQ) on rats erytrocyte and tissue Malondialdehid (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) activities after DMN injection. The results are means +S.EM. The group means were compared with one-way ANOVA with post-hoc LSD test
Discussion
Cirrhosis can be defined as diffuse fibrosis with hepatocyte regeneration [6]. Initially cirrhosis was considered as irreversible condition due to liver damage, recently it is regarded as partly reversible process [1].
During liver fibrosis, scar tissue may appear, and hepatic stellate cells (HSC) are responsible for this production. Rapid changes in ECM composition and damaged hepatocytes, Kupffer and endothelial cells can be counted among the stimuli that induce HSC activation [20]. Free radicals and lipid peroxides also play a role in the prognosis of hepatic damage and in some liver diseases that result in fibrosis. Free radicals that form under certain physiological conditions can be eliminated through antioxidant mechanisms [21].
In recent years, there has been a tendency to use natural products to treat some diseases as an alternative to classical treatments [22]. In relevant studies, NS has been reported to be potent and protective against the effects of a variety of hepatotoxic and genototoxic agents, such as tert-butyl, hydrogen peroxide, carbon tetrachloride (CC14), doxorubicin and cisplatin [22]. In addition, in in vivo and in vitro studies in rats, TQ was found to prevent oxidative damage [23].
TQ acts as an antioxidant in the tissues. It is assumed that this effect is related to thromboxane B2 and the production of the enzyme leukotriene B4 (by inhibiting cyclooxygenases and 5-lipoxygenase, respectively) and the inhibition of the peroxidation of membrane lipids [23].
The critical phase where hepatocyte necrosis will take place when exposed to oxidative stress has been identified as the increasing plasma membrane bleb formation. The mechanism put forward for the increasing bleb formation is the increase in thromboxane B2. Since TQ inhibits the production of lipid peroxidation, and eicosanoids such as thromboxane B2 and leukotriene B4, it is assumed that it also protects the liver with its inhibitor effect on thromboxane B2 production [13]. It has also been stated that NSO is more effective than TQ as an inhibitor in lipid peroxidation and the formation of eicosanoids [24].
The generations of fibrosis models in rats are realized through hepato-toxic agents such as DMN and CCl4 [25]. Türkdoğan et al. reported that in prevention of liver damage triggered by CCl4, NS hindered fibrosis-related coagulation necrosis and hydrophilic degeneration by inhibiting lipid peroxidation and also through immune-modular activity. The results of the current study are in agreement with Türkdoğan et al [1]. In our study, incomplete cirrhosis was formed in the rats injected with DMN. Fibrotic expansion with/without septa was observed in some portal areas of the livers of the rat groups treated with NS, NSO and TQ.
In one study, Abdal-Wahhab et al. showed that NSO and TQ decrease oxidative stress and thus preventing liver damage [26]. In our study, compared to the DMN group, we determined that there were significant decreases in ALT, AST, GGT, ALP, TBIL, and DBIL levels of the other groups. However, in the NS group, the decrease in ALT, AST, GGT, and ALP levels was lower compared to the TQ and NSO groups. This finding can result from incomplete intake of NS mixed with food by rats.
The TBIL and DBIL levels of the TQ group were found to be significantly lower compared to NS and NSO groups. These results indicated that TQ was more active in bilirubin metabolism. Because of serious damage of the parenchyma in cirrhosis, synthesis of liver functions decreases, as with albumin which reflects synthesizing capacity of hepatic parenchymal cells.
In accordance with the experimental study by Mahmoud et al, we found that total BIL and Alb levels in the NSO group were statistically significantly higher compared to the TQ and NS groups [27]. Compared to the DMN group, increase in capacity of synthesizing was interpreted as an indication of the protective effects of the components, and indicated that liver fibrosis was reversed. In liver cirrhosis, the albumin/globulin ratio, normally 1.3-1.8, is reversed. Although an increase in Alb synthesis capacity has been reported, the effects of NS and its components have not been researched in terms of these parameters. Compared to the other groups, a significant increase in the albumin/globulin ratio in the NSO group has been detected. The increase in the Alb levels in the NSO group result from the increase in the liver synthesis capacity caused by the components of NSO. Free radicals and lipid peroxides are also factors in the pathogenesis of certain liver diseases resulted from hepatic damage and fibrosis [28]. It is reported that TQ plays a potential role in the inhibition of the production of ROS causing lipid peroxidation [13,29,30]. In another study, Yildiz et. al. showed that NS has protective effects against liver ischemia-reperfusion damage [23]. Besides, Kanter et al. reported that NS increases activation of the antioxidant protection system, preventing lipid peroxidation induced by CCl4 and liver damage in rats [21]. It is also reported in related studies that the NS and its components have protective effects over induced hepatotoxicity. Yet, no experimental study has been conducted so far that NS and its components have reversed fibrosis. For this reason, we researched the anti-fibrotic effects of NS, NSO and TQ in DMN-induced liver fibrosis. To the best of our knowledge, this study is the first to investigate the effect of NS and its components on experimental liver fibrosis. We found that tissue and erythrocyte MDA levels in the DMN group were significantly increased, whereas tissue and erythrocyte SOD and GSH-Px activities were significantly decreased compared to those of the other groups. In addition, a significant increase was also detected in tissue SOD and GSH-Px activities while a significant decrease in tissue MDA levels was determined in the NSO group compared to the TQ and NS groups. The reason for more antioxidant effect of NSO compared to the TQ and NS groups may be related to the antioxidant ingredients of NSO other than TQ, such as carvakrol, tanethole, 4-terpineol, or to the conversion of the dihydrothymoquinone diaphoresis enzyme into dihydrothymoquinone metabolites, a stronger antioxidant than TQ. While there appeared to be significant differences in SOD, GSH-Px activities and MDA levels in the liver tissues between the NSO and the TQ and NS groups, we found no difference in terms of erythrocytes between the NSO and the TQ groups.
As a result, in this study, we investigated the effects of NS and its components on the oxidant/antioxidant system in a model of experimental liver fibrosis of rats. The mechanism by which NS and its components reversed liver fibrosis has not been precisely understood yet. This study is promising that the use of NS and its components can also be used in treatment of fibrosis in humans. Our results also showed that by reducing the formation of MDA, an indicator of lipid peroxidation, and increasing antioxidant enzyme activities, and improving liver function tests, NS, NSO and TQ had the antioxidant effects and a free radical scavenging activity and reduced oxidative stress conditions in the erythrocyte and liver tissue in a model of experimental liver fibrosis of rats. However, further experimental studies are needed to explain the molecular mechanism of NS, NSO and TQ protective effects.
Conflict of interest
The authors have no conflict of interest.
Funding
No funding was received for this project.
Conflict of interest statement & statement of authorship sections
E.S, M.T, V.S. and S.T contributed to the design of the study. Data collection was carried out by E.S, V.S, S T and Y.B. Data analysis was done by E.S, M.T, M.K and S.T. The manuscript was written by E.S., V.S., M.T.G. and S.T. all provided significant advice or consultation. The final manuscript was approved by all authors.
Acknowledgments
The authors thank Professor Cahit BAĞCI for his research assistance.
References
- Turkdogan MK, Ozbek H, Yener Z, Tuncer I, Uygan I, Ceylan E. The role of Urticadioica and Nigella sativat heprevention of carbon tetrachloride induced hepatotoxicity in rats. PhytotherRes. 2003;17:942–6.
- Bonis PA, Friedman SL, Kaplan MM. Is liver fibrosis reversible? N Engl J Med. 2001;344:452–4.
- Bhattacharjee A, Lappi VR, Rutherford MS, Schook LB. Molecular dissection of dimethylnitrosamine (DMN)- induced hepatotoxicity by Mrna differential display. Toxicol Appl Pharmacol. 1998;150:186-95.
- Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719–27.
- Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis seminars in liver disease. 2001;3:351-72.
- Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. Journal of Hepatology 2012; 56: 1171–1180.
- Friedman SL. Molecularregulation of hepaticfibrosis, an integrated cellula rresponsetotissue injury. J BiolChem. 2000;275: 2247–50.
- Taysi S. Oxidant/antioxidant status in liver tissue of vitamin B6 deficient rats. Clin Nutr.2005;24:385-9.
- Halliwell B, Gutteridge JMC. Freeradicals in biology and medicine (3nd ed).Oxford, Oxford University, 1999:30–90.
- Fararh KM, Shimizu Y, Shiina T, Nikami H, Ghanem MM, Takewaki T. Thymoquinone reduces hepatic glucose production in diabetic hamsters.Research in Veterinary Science 2005;79:219-23
- Kanter M, Çoşkun Ö, Budancamanak M. Hepato protective effects of Nigellasativa L and Urticadioica Lon lipid peroxidation, antioxidant enzym esystemsandliver enzymes in carbontetrachloride-treatedrats. World J Gastroenterol. 2005;11:6684–88.
- Kanter M. Effects of Nigella sativa and its major constituent, Thymoquinone on sciaticnerves in experimental Diabetic Neuropaty. NeurochenRes. 2008;33:87–98.
- Daba MH, Abdel-Rahman MS. Hepatoprotective activity of thymoquinone in isolated rat hepatocytes. ToxicolLett. 1998;95:23–9.
- Jain SK, Mcvie R, Duett J, Herbst JJ. Erythrocyte membrane lipid peroxidation and glycocylated hemoglobin in diabetes. Diabetes. 1989;38:1539–43.
- Okawa H, Ohishi N, Yagi K. Reaction of linoleic acid hydroperoxide with thiobarbituri casid. J Lipid Res. 1978;19:1053–57.
- Sun Y, Oberley LW, Li YA. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988; 34: 497–500.
- Paglia DE, Valentina WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169
- Bradford MM. Arapid and sensitive method fort hequantitation of microgram quantities of protein utilizing the principle of protein-dyebinding. Anal Biochem. 1976; 72: 248-254.
- Ishak K, Baptista A, Bianchi L, Callea F, DE Groote J, Gudat F. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22: 696-99.
- Urtasun R, Nieto N. Hepatic stellate cells and oxidative stres. Rev Esp Enferm Dig. 2007;99:223–30.
- Kanter M. Effects of Nigella sativa L. and Urtica diocica L. on lipid peroxidation, antioxidant enzyme systems and some liver enzymes in CCl4 –treated rats. J Vet Med. 2003;50:264–8.
- Salem ML. Immunomodulatory and immune therapeutic properties of the Nigell asativa L seed. Intimunopharmacol. 2005;5:1749–70.
- Yildiz F, Çoban S, Terzi A, Ateş M, Aksoy N, Ocak AR, Bitiren M. Nigella sativa relieves the deleterious effects of ischemia reperfusion injury on liver. World J Gastroenterol. 2008;14:504–20.
- Houghton PJ, Zarka R, Hoult JR. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;6:33–6.
- Bhattacharjee A, Lappi VR, Rutherford MS, Schook LB. Molecular dissection of dimethylnitrosamine (DMN) induced hepatotoxicity by Mrna differential display. Toxicol Appl Pharmacol. 1998;150:186-95.
- Abdel-Wahhab MA, Aly SE. Antioxidant property of Nigella sativa (blackcumin) and Syzgiumaromaticum (clove) in rats during aflatoxicosis. J Appl Toxicol. 2005;25:218–23.
- Mahmoud MR, El-abhar HS, Saleh S. Theeffect of Nigella sativa oil against the liver damage induced by Schistosoma mansoni infection in mice. J Ethnopharmacol. 2002;79:1–11.
- Nieto N, Friedman SL, Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology. 2002;35:62–73.
- Mansour MA. Protectiveeffects of Thymoquınone and Desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sciences. 2000;66:2583–91.
- Nagi MN, Alam K, Badary OA, Al Shabanah OA, Al-Sawaf HA, Al- Bekair I. Thymoquinone protects against carbon tetrachloride hepatotoksixity in micevia an antioxidant tmechanism. Biochem Mol Biol Int. 1999; 47: 153–159