Research Article - Biomedical Research (2017) Volume 28, Issue 17
Effect of minocycline on vascular proliferation after corneal alkaline burn: A mechanism study
Haijun Wu1*, Xin Dai2, Hui Li1 and Chunying Lv1
1Department of Ophthalmology, Heze Municipal Hospital, Heze, Shandong, PR China
2Shandong Heze Medical College, Heze, Shandong, PR China
Accepted date: August 21, 2017
Abstract
Impairment of vision or blindness always occurs in the progress of Corneal Neovascularization (CNV). CNV may cause corneal graft rejection at corneal transplant status. Thus, intervention of CNV is of concern to clinical treatment. Given minocycline was reported with inhibiting effect on CNV, our study was focused on specific effect of minocycline on CNV after corneal alkaline burn. Seventy five male Sprague Dawley rats (12 w old) were randomly and equally assigned into three groups, receiving Phosphate-Buffered Saline (PBS) (control group), 60 mg/kg or 30 mg/kg minocycline, respectively. Sodium hydroxide (NaOH) solution was used to establish corneal alkaline burn rat model, which were performed on left eyes of experimental rats. Vascular cover area of corneal hyperplasia was assessed for three groups at 5 d, 10 d and 15 d after corneal alkaline burn, respectively. Vascular proliferation was analysed for three groups. Expressions of VEGF and VEGFR were examined with qRT-PCR at 15 d after corneal alkaline burn. HUVECs were cultured with different concentration of minocycline. HUVECs proliferation, AKT and phosphorylation level of ERK1/2 were assessed in vitro, respectively. Compared with control group, CNV area was significantly decreased in minocycline treated groups (p<0.05). qRT-PCR showed that expressions of VEGF and VEGFR were inhibited by minocycline treatment. Cell experiment on HUVECS demonstrated that proliferation was inhibited after minocycline treatment, and such inhibiting effect was dose-dependent. In addition, minocycline decreased expression of AKT, and reduced phosphorylation level of ERK1/2. Minocycline inhibited vascular proliferation of CNV after alkaline burn via down-regulating multiple factors, including VEGF, VEGFR, AKT and phosphorylation level of ERK1/2.
Keywords
Minocycline, Corneal alkaline burn, CNV, Proliferation
Introduction
Corneal is a hyaline membrane without vascular structure under normal condition, while CNV was induced due to stimuli of inflammation, infection, trauma or degenerative change [1,2]. Corneal alkaline burn always resulted in severe CNV, which may cause blindness or corneal graft rejection at corneal transplant status [3]. Thus, it is a hot topic how to inhibit CNV after corneal alkaline burn.
The tetracyclines were originally identified as broad-spectrum antibiotics and can be classified as natural products, semisynthetic compounds and chemically modified tetracyclines. The bacteriostatic effect of the tetracyclines is reported to be mediated by inhibition of protein synthesis through preventing the attachment of the aminoacy1 t-RNA to the ribosome in the bacterial cell [4]. Therefore, tetracycline has been used extensively for prophylaxis and therapy in both human and animal infections. However, recent studies have demonstrated that tetracycline and its analogs doxycycline, minocycline, and tigecycline, have several anti-inflammatory properties [5]. Animal study has proved that doxycycline inhibited CNV [6], while other studies have demonstrated the non-antibiotic properties of minocycline, such as anti-inflammatory and anti-apoptotic activities as well as inhibition of proteolysis, angiogenesis, and tumor metastasis [7,8]. Furthermore, in vitro studies demonstrated that minocycline can decrease human umbilical vascular endothelial cell proliferation and tube formation, tumor cell migration, inducible nitric oxide synthetase expression, and induce macrophage apoptosis [9,10]. However, whether minocycline affects vascular proliferation after corneal alkaline burn remains poorly understood. Our study aimed to explore the effect of minocycline on vascular proliferation after corneal alkaline burn, and potential mechanisms underlying minocycline treatment on CNV.
Materials and Methods
Experimental animals
75 male Sprague Dawley rats (12 w old) were purchased from Laboratory Animal Centre of Shandong University (Jinan, China). All rats were bred under normal situation (temperature 25°C, humidity 60 ± 10%). Corneal alkaline burn models were performed on left eyes of experimental rats. Rats in three groups were treated with PBS (10 ml/kg, control), 60 mg/kg minocycline (Sigma) or 30 mg/kg minocycline, respectively.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of Heze Municipal Hospital (Heze, China).
Corneal alkaline burn models
Rats were anaesthetized with trichloroacetaldehyde (60 mg/ kg). NaOH solution (0.1 M, 3 μL) was added on circular filter paper (diameter 3 mm). Cover NaOH-treated filter paper on corneal center of left eye for 40 s, then rinse left eye twice with normal saline [11].
Evaluation of CNV
Revascularization and epithelium injury were assessed for burned eyes at 5 d, 10 d and 15 d after corneal alkaline burn. Photographing was performed with microscope to evaluate CNV. Pixel analysis software was used to assess CNV area and injury ratio (CNV area/Corneal area × 100%) with routine protocol [12].
qRT-PCR
Rats were anaesthetized with trichloroacetaldehyde (60 mg/kg) at 15 d after corneal alkaline burn. Eyeballs were harvested for extraction of corneal. RNA was extracted from a small part of corneal with RNA extraction kit (QIAGEN). mRNA expressions of VEGF and VEGFR were examined with RTPCR. RT-PCR conditions are as follows: 95°C/3 min, 95°C/15 s, 60°C/30 s (40 cycles). β-actin was used as internal control. 2-ΔΔCt method was performed to analyse RT-PCR [13]. Primer sequence was showed in Table 1.
| Name | Sequence | |
|---|---|---|
| β-actin | F | 5'-ATCTGGCACCACACCTTC-3' |
| R | 5'-AGCCAGGTCCAGACGCA-3' | |
| VEGF | F | 5'-TTACTGCTGTACCTCCACC-3' |
| R | 5'-ACAGGACGGCTTGAAGATG-3' | |
| VEGFR | F | 5'-GTGATCAGCTCCAGGTTTGACTT-3' |
| R | 5'-GAGGAGGATGAGGGTGTCTATAGGT-3' | |
Table 1: Primer sequence.
Immunohistochemistry
Rats were anaesthetized with trichloroacetaldehyde (60 mg/kg) at 15 d after corneal alkaline burn. Eyeballs were harvested for extraction of corneal. Paraffin embedding was performed on corneal for further paraffin section. Deparaffinage, antigen retrieval and block of non-specific antibody binding sites were performed on paraffin slice with routine protocol [13]. Results of chromogenic reaction were observed with microscope.
Cell culture
HUVECs were purchased from American Type Culture Collection (Manassas, VA, USA). HUVECs were cultured in hormone-laden epithelial cell medium (DMEM-F12 medium with 10% Fetal Bovine Serum, 5 μg/ml bovine insulin and 10 ng/ml EGF). HUVECs were transferred into 96-well plate (200 μL/well). Experimental wells were treated with 20 mg/L, 40 mg/L, 80 mg/L and 160 mg/L minocycline, while control wells were treated with normal saline.
MTT assay
MTT solution was added into 96-well plate (70 μL/well) 20 h after minocycline or normal saline treatment. Maintain reaction for 4 h. Remove the medium and add DMSO (150 μL/well). Microplate reader was used to examine absorbance value (A) at 490 nm. Growth curve was established with absorbance values. Growth inhibition ratio was calculated with following formula: inhibition ratio (%)=(Acontrol-Aexperimental)/Acontrol × 100% [14].
Western blot
Cell lysis was performed for minocycline-treated HUVECs. Total protein was extracted for SDS-PAGE electrophoresis. Primary antibodies were pAKT antibody and pERK1/2 antibody. β-actin was used as internal control. Gel imaging system was used for analysis of specific protein bands [15].
Statistical analysis
SPSS 20.0 software was used for data processing. Measurement data are normal distribution to mean ± Standard Deviation (SD). One-Way ANOVA was performed for statistical significance. P value<0.05 was considered to be statistically significant.
Results
Inhibiting effect of minocycline on CNV after corneal alkaline burn
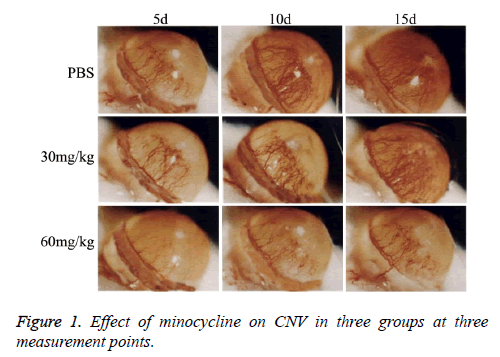
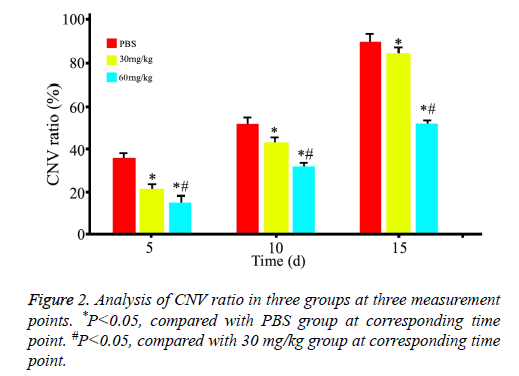
As showed in Figure 1, CNV was observed in all rats with corneal alkaline burn. CNV ratio (area of CNV/area of corneal) was calculated and showed in Figure 2. Area of CNV gradually increased after corneal alkaline burn. Compared with normal saline treated rats, 30 mg/kg minocycline treated rats had a significant smaller area of CNV (P<0.05) at 5 d and 10 d, without at 15 d. Moreover, 60 mg/kg minocycline treated rats had the smallest area of CNV (P<0.05) at three time points, suggesting minocycline slowed down the progress of CNV in dose-dependent manner.
Minocycline decreased expression of VEGF and VEGFR
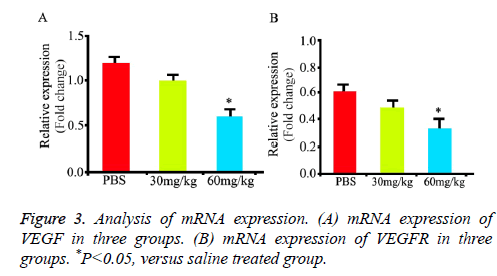
Compared with normal saline treated rats, minocycline treated rats had lower mRNA expression of VEGF (Figure 3A, P<0.05) at 15 d. Compared with normal saline, minocycline-treated rats had lower mRNA expression of VEGFR (Figure 3B, P<0.05) at 15 d. These results suggested minocycline decreased expression of VEGF and VEGFR.
Minocycline inhibited proliferation of HUVECs
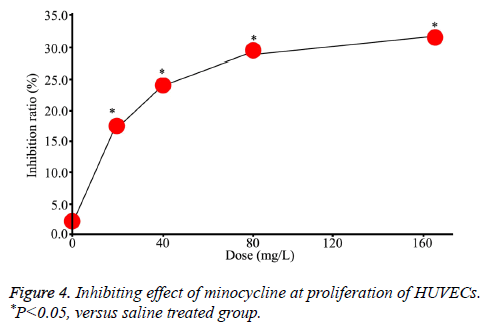
MTT assays showed that the proliferation of HUVECs was inhibited after minocycline treatment and the inhibitory effect was enhanced with increased dose, suggesting such effect was dose-dependent (Figure 4).
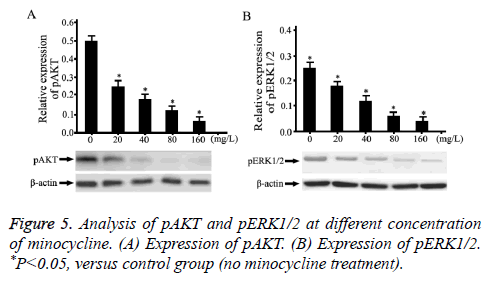
Minocycline decreased phosphorylation levels of AKT and ERK1/2
Phosphorylation levels of AKT and ERK1/2 (pAKT and pERK1/2) was detected by Western-Blot (Figure 5). Compared with normal saline, minocycline-treated HUVECs reduced the expression of pAKT in a dose dependent manner (Figure 5A, P<0.05). Compared with normal saline, minocycline-treated HUVECs decreased the expression of ERK1/2 in a dose dependent manner (Figure 5B, P<0.05).
Discussion
As a semisynthetic tetracycline, minocycline was widely used in clinical treatment [16]. With intense researches, minocycline not only has functions against multiple cellular events [17], including apoptosis, oxidation and inflammation, but also plays a part in many biological activities, such as protection of central nervous system, inhibition of tumor angiogenesis [18,19]. Previous study showed that median inhibitory concentration of minocycline was 310 mg/kg for rats, while our used 60 mg/kg as maximum concentration in vivo, suggesting minocycline used in our study was still in normal range. Our study explored the potential mechanism underlying the effect of minocycline on vascular proliferation after corneal alkaline burn.
There are two main phases of CNV after corneal alkaline burn. At first phase, endothelial proliferation related genes are over expressed, including VEGF, VEGFR and bFGF. Components of extracellular matrix change and multiple cytokines (MMPs, IL-1) are activated [20]. Our study demonstrated that minocycline significantly decreased expression of VEGF and VEGFR in corneal tissue, verified by qRT-PCR, which was consistent with a previous in vivo study showing minocycline-treated mice displayed reduced mRNA expression of VEGFR1 and VEGFR2 [21] and an in vitro study revealing reduced VEGF mRNA expression in minocycline-treated human renal cell carcinoma cell lines SMKT-R-1 cells [22]. As is well-known, VEGF and VEGFR are strongly linked to angiogenesis, thus minocycline attenuated CNV via decreasing VEGF and VEGFR. In addition, minocycline also down-regulated phosphorylation levels of AKT and ERK1/2, suggesting minocycline inhibited proliferation in CNV via decreasing pAKT and pERK1/2.
As an important protein related to apoptosis and proliferation, Akt need be activated through phosphorylation so as to transduce proliferation signal via PI3K/Akt pathway. On the other hand, Akt inhibited apoptosis via enhancing phosphorylation of pro-apoptotic protein BAD, which was inactivated through phosphorylation [23]. What’s more, Akt shortens the cell cycle and promoted cell division by decreasing expression cyclin-dependent kinase inhibitor proteins p27kip1 [24]. ERK1/2 is the downstream effector in RAS pathway. Activated pERK1/2 enters into the nucleus to activate transcription factors, which promoted proliferation and differentiation [25]. Our study showed that minocycline inhibited proliferation via abrogating activation of pAKT and pERK1/2, consistent with a previous study demonstrating inhibition of vascular endothelial growth factor-induced smooth muscle cell migration by minocycline through downregulation of ERK1/2 and PI3K/Akt pathway phosphorylation [26].
In summary, minocycline has a promising effect on inhibition of CNV after corneal alkaline burn, and maintains normal function of corneal as ocular Media. Minocycline is a potential drug for corneal alkaline burn in clinical scenario.
Conclusion
Minocycline inhibits vascular proliferation in CNV after corneal alkaline burn via multiple mechanisms, such as decreasing expression of VEGF and VEGFR, inhibiting phosphorylation of Akt and ERK1/2.
References
- Bachmann B. The association between corneal neovascularization and visual acuity: a systematic review. Acta Ophthalmologica 2013; 91: 12-19.
- Abdelfattah NS. Clinical correlates of common corneal neovascular diseases: a literature review. Int J Ophthalmol 2015; 8: 182-193.
- Han Y. Therapeutic effects of topical netrin-4 inhibits corneal neovascularization in alkali-burn rats. PloS One 2015; 10: 0122951.
- Chopra I. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 2001; 65: 232-260.
- Tilakaratne A. Anti-inflammatory actions of adjunctive tetracyclines and other agents in periodontitis and associated comorbidities. Open Dent J 2014; 8.
- He L. Doxycycline inhibits polarization of macrophages to the proangiogenic M2-type and subsequent neovascularization. J Biol Chem 2014; 289: 8019-8028.
- Garrido-Mesa N, Zarzuelo A, Galvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol 2013; 169: 337-352.
- Pourgholami MH. Minocycline inhibits malignant ascites of ovarian cancer through targeting multiple signaling pathways. Gynecol Oncol 2013; 129: 113-119.
- Fife RS, Sledge GW, Sissons S, Zerler B. Effects of tetracyclines on angiogenesis in vitro. Cancer Lett 2000; 153: 75-78.
- Bettany JT, Wolowacz RG. Tetracycline derivatives induce apoptosis selectively in cultured monocytes and macrophages but not in mesenchymal cells. Adv Dent Res 1998; 12: 136-143.
- Uchiyama M. An ophthalmic solution of a peroxisome proliferator-activated receptor gamma agonist prevents corneal inflammation in a rat alkali burn model. Mol Vis 2013; 19: 2135-2150.
- Bignami F. Corneal alkali burn induces inflammatory changes in the central nervous system. Investig Ophthalmol Vis Sci 2013; 54: 3583-3583.
- Kimber NE. Skeletal muscle fat and carbohydrate metabolism during recovery from glycogen-depleting exercise in humans. J Physiol 2003; 548: 919-927.
- Wei JT. Oleanolic acid inhibits proliferation of HUVECs, and inhibits migration and tube formation via VEGF pathway. Acta Pharmaceutica Sinica 2012; 47: 1457-1462.
- Yang L. Inhibitory effects of polysaccharide extract from Spirulina platensis on corneal neovascularization. Mol Vis 2009; 15: 1951-1961.
- Lang N. Minocycline exerts acute inhibitory effects on cerebral cortex excitability in humans. Epilep Res 2013; 107: 302-305.
- Garrido-Mesa N, Zarzuelo A, Galvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol 2013; 169: 337-352.
- Abcouwer SF. Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. J Neuroinflamm 2013; 10: 149.
- Sakata H. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci 2012; 32: 3462-3473.
- Tian S. Review of the progress in corneal neovascularization animal models. Am J Biochem Biotechnol 2015; 11: 221-232.
- Xiao O, Xie ZL, Lin BW, Yin XF, Pi RB. Minocycline inhibits alkali burn-induced corneal neovascularization in mice. PLoS One 2012; 7: 41858.
- Sasamura H. Inhibitory effect on expression of angiogenic factors by antiangiogenic agents in renal cell carcinoma. Br J Cancer 2002; 86: 768-773.
- Koh PO. Ferulic acid prevents the cerebral ischemic injury-induced decrease of Akt and Bad phosphorylation. Neurosci Lett 2012; 507: 156-160.
- Tan W. PTEN/Akt-p27kip1 signaling promotes the BM-MSCs senescence and apoptosis in SLE patients. J Cell Biochem 2015; 16: 1583-1594.
- Selvaraj N, Budka JA, Ferris MW, Jerde TJ, Hollenhorst PC. Prostate cancer ETS rearrangements switch a cell migration gene expression program from RAS/ERK to PI3K/AKT regulation. Mol Cancer 2014; 13: 61.
- Yao JS. Minocycline exerts multiple inhibitory effects on vascular endothelial growth factor-induced smooth muscle cell migration: the role of ERK1/2, PI3K, and matrix metalloproteinases. Circ Res 2004; 95: 364-371.