Research Article - Biomedical Research (2017) Volume 28, Issue 21
Effect of mangiferin on brain inflammation in rats with spontaneous hypertension
Zhou Su1, Yufang Zhan2, Yanxia Song3 and Guiying Zhang4*
1Clinic Center, Rizhao People’s Hospital, Rizhao, Shandong, PR China
2Department of Pharmacy, Rizhao City Shexian People's Hospital, PR China
3Yanxia Song, Department of Pharmacy, Rizhao City Dermatology Prevention and Control, PR China
4Department of Pharmacy, Rizhao People’s Hospital, Rizhao, Shandong, PR China
Accepted date: December 11, 2017
Abstract
Objective: To study the protective mechanism of mangiferin on brain inflammatory lesion in spontaneously hypertensive rats.
Methods: Twenty rats with spontaneous hypertension were randomly and evenly divided into model group and mangiferin group. Ten more male rats of the same age were selected as control group. After 2 months of drug infusion, morphological changes of brain in rats with spontaneous hypertension were observed. Meanwhile, levels of TNF-α, IL- 1β and ICAM-1 in the brain of those rats were measured by ELISA. Western blot assay was performed to detect the expression of Monocyte Chemoattractant Protein (MCP-1) and Cell Chemokine Receptor (CCR2) protein.
Results: The contents of TNF-α, IL-1β and ICAM-1 in the model group increased significantly when compared with the normal control group and were markedly higher than those in the mangiferin group. The results of Western blot assay showed that the expressions of MCP-1 and CCR2 in the model group increased significantly compared with the normal control group and were markedly higher than those in the mangiferin group.
Conclusion: Mangiferin can effectively reduce levels of inflammatory factors such as TNF-α, IL-1β and ICAM-1 in brain tissues of spontaneously hypertensive rats. It has obvious anti-inflammatory effect and its mechanism may be related to the inhibition of expression of MCP/CCR2 signaling pathway.
Keywords
Mangiferin, Spontaneous hypertensive rats, Brain tissue, TNF-α, IL-1β, MCP/CCR2 signaling pathway
Introduction
Spontaneous hypertension is a chronic inflammatory disease characterized by activation of lymphocyte and monocyte system [1]. Research shows that [2] there exists interaction between inflammation and hypertension: on one hand, by stimulating the expression of some factors like TNF-α, IL-1β and ICAM-1 hypertension can cause the activation of proinflammatory state; which in turn promotes the development of hypertension and leads to vascular remodeling as well as the injuries of target organs like brain. Other studies have shown that [3,4] spontaneously hypertensive rats suffer many inflammatory lesions in the heart, brain and kidney. Mango leaves contain abundant medicinal herbs. Mangiferin, a tetrahydroxy milrinone carbon glycoside also known as chimonin, is a double benzene, pyridine ketone compound and is the main active ingredient of mango leaves existing in many plants. It has been reported that [5,6] mangiferin has the effects of scavenging lipid peroxide in brain tissue and reducing its damage to neurons so as to protect the normal function of neurons. Mangiferin, with similar anti-inflammatory function to glucocorticoid, can reduce capillary permeability and has a protective effect on myocardial ischemia reperfusion injury in rats. These studies suggest that mangiferin may protect against cerebral ischemia reperfusion injury. However, there are few reports on whether mangiferin can reduce the levels of inflammatory factors in brain tissues of spontaneously hypertensive rats. In this study with spontaneously hypertensive rats as the animal model of hypertension, we observed the effect of mangiferin on the expressions of such inflammatory factors as TNF-α, IL-1β and ICAM-1 in brain tissues of such rats as well as on MCP/CCR2 signaling pathway, which will help to further understand the protective effect of mangiferin on inflammatory injury of this sort and its mechanism to provide experimental evidence for developing the new drug of mangiferin.
Materials and Methods
Experimental animals
A total of 20 male spontaneous hypertension rats and 10 male rats, all aged 10 weeks, were purchased from Beijing Weitong Lihua Experimental Animal Technology Co. Ltd, with the body weight of 265-330 g. During the rearing of laboratory animals, unlimited food and water were provided with controllable temperatures, humidity and light conditions.
Major drugs and reagents
Mangiferin was extracted from mango leaves in the Anacardiaceae plants and provided by Shanghai Yuduo Biotechnology Co. Ltd. ELISA kit for detecting Tumor Necrosis Factor alpha (TNF-α), IL- 1β and Intercellular Adhesion Molecule-1 (ICAM-1) was provided by Nanjing Bejme Sen Biotechnology Co. Ltd; CCR2, MCP-1 first antibody was provided by Shanghai Hui Ying Biotechnology Co. Ltd.; and other reagents used in the experiment were all analytically pure.
Experimental method
Twenty rats with spontaneous hypertension were randomly and evenly divided into model group and mangiferin group. Another ten male rats of the same age were selected as control group.
Administration
The rats in the model group and the control group were given injection equal volume of sterilized water, while the mangiferin group was injected with mangiferin (30 mg/Kg/d). All animals were administered at 10 ml/Kg/d with drug withdrawal successive 2 months after the administration and the rats were fasted for 12 h before being killed.
Sample collection
Two months after the experiment, the rats were injected with 10% chloral hydrate at the dose of 3 ml/kg for anesthesia and skull was opened to obtain the brain tissue for morphological observation, double antibody sandwich Enzyme-Linked Immunosorbent Assay (ELISA) detection and Western blot analysis.
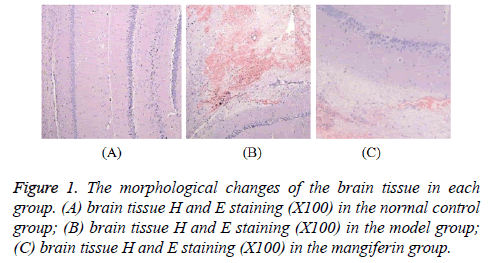
Morphological observation
The brain tissue was fixed with 4% paraformaldehyde and washed with tap water for overnight before dehydration followed by ethanol dehydration at all levels with automatic dehydrator, being made to be transparent with clear liquid, being soaked with paraffin twice and routinely paraffinembedded with embedding machine; the section of 5 μm was incised with pulley section cutter followed by Hematoxylin and Eosin (H and E) staining, being made to be transparent with clear liquid and sealed with neutral balsam for microscopic examination.
The detection of TNF-α, IL- 1β and ICAM-1 contents in brain tissues of rats by ELISA
The contents of TNF-α, IL-1β and ICAM-1 in brain tissue of rats were examined by ELISA strictly in accordance with the instructions.
The detection of MCP-1 and CCR2 expression in brain tissues of rats by Western blot
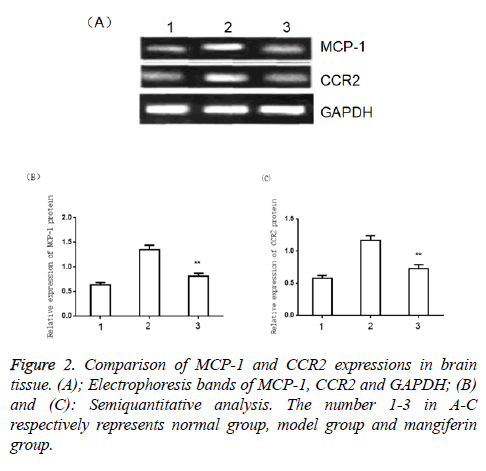
The frozen brain tissue with the total weight of 200 mg was collected and homogenized in 2 ml PBS (pH7.4) containing protease inhibitors followed by 2-time centrifugation for 15 min at 4°C with the supernatant as the total protein sample. After the determination of protein concentration by Lowry, the sample was loaded with 50 μg protein per hole. Polyacrylamide Gel Electrophoresis (SDS-PAGE) was conducted for separation of total protein followed by electrophoretic transfer of the protein to cellulose nitrate membrane and being closed for 1 h with 5% skim milk at room temperature, then the first antibodies MCP-1 (1:1000), CCR2 (1:1000) and GAPDH (1:5000) were respectively added at 4°Cfor the overnight. After PBS-T washing for 3 × 5 min, the sample was incubated for 1 h at room temperature with corresponding horseradish peroxidase labeled second antibody (1:5000), the chemiluminescence method was used for chromogenic reaction was and finally the sample was developed and fixed in the darkroom.
Statistical processing
Data analysis was carried out on SPSS 21 statistical software. All the data were expressed as mean ± standard deviation and continuous variables were tested by one-way ANOVA. P<0.05 suggests the difference is statistically significant.
Results
Comparison of morphological finding of brain tissue in all groups
No obvious change was seen in the normal control group and in the model group there was local wedge type laceration with hemorrhage foci as well as the damages in polymorphic layer of cortex and subarachnoid hemorrhage on the surface of contused areas; in addition to visible cell necrosis, in some areas parts of nerve cell has smaller body as well as condensed cytoplasm and nucleus, showing morphological changes of apoptosis. Changes in the mangiferin group were similar with those in the model group but with lower degree of lesion as shown in Figure 1.
Comparison of contents of inflammatory factors in brain tissue
The contents of TNF-α, IL-1β and ICAM-1 in the model group increased significantly (P<0.05) when compared with the normal control group and were markedly higher than those in the mangiferin group (P<0.05), as shown in Table 1.
| Group | n | TNF-α (ng/L) | IL- 1β (ng/L) | ICAM-1 (ng/L) |
|---|---|---|---|---|
| Normal control group | 10 | 8.34 ± 0.53 | 1.76 ± 0.74 | 18.87 ± 3.26 |
| Model group | 10 | 16.72 ± 2.15 | 4.58 ± 0.33 | 44.12 ± 4.05 |
| Mangiferin group | 10 | 11.68 ± 1.72 | 2.03 ± 0.55 | 23.26 ± 2.38 |
| F | 11.73 | 10.00 | 12.94 | |
| p | 0.033 | 0.027 | 0.029 |
Table 1. Comparison of contents of inflammatory factors in brain tissue.
Comparison of MCP-1 and CCR2 expressions in brain tissue
The results of Western blot assay showed that the expressions of MCP-1 and CCR2 in the model group increased significantly compared with the normal control group (P<0.05) and were markedly higher than those in the mangiferin group (P<0.05), as shown in Figure 2.
Discussion
Essential hypertension is one of the most common cardiovascular diseases and a risk factor for stroke, coronary heart disease as well as heart failure. Its pathogenesis is complicated and is commonly considered because of the synthetic action of various acquired factors based on certain genetic susceptibility [7]. In recent years, it has been found and confirmed that inflammatory factors play an important role in the occurrence, development and prognosis of hypertension. Therefore, the study of hypertension from inflammatory point of view has become a hot spot in the field of medicine. Inflammation is a common pathological phenomenon and frequently results from hematoma as well as its mechanical compression [8]. Inflammatory response, however, is a necessary condition for secondary brain injury. It, on the one hand, helps the host to remove and absorb necrotic tissues as well as cell debris with the effect of repairing injury as a normal defense response, on the other hand, it will give rise to release of various enzymes/inflammatory mediators and induce edema around brain tissue with worse damages to nerve cells.
Inflammatory factor refers to a group of small peptides with wide range of biological activities and synthesized and secreted by body's immune cells (monocytes/macrophages and lymphocytes and so on) as well as non-immune cells (epidermal cells, vascular endothelial cells, fibroblasts and so on) with the function of regulating inflammation and immune response of varied cells. The main inflammatory factors associated with hypertension are TNF-α, IL-1βand ICAM-1 [9]. Mangiferin can inhibit the inflammatory response mediated by mast cells and control the amount of peanut four acid in macrophages with a good curative effect on peanut four acid-induced inflammatory auricle edema in mice [10]. Pharmacological experiments show that [11] mangiferin has anti-inflammatory and expectorant effects. Rivera as well as his colleagues has conducted a study with allergic asthma mouse model as the objects [12] and found that mangiferin has anti-inflammatory effect by its function to alleviate airway inflammation in allergic asthmatic mice. The results of this study show that the contents of TNF-α, IL- 1β and ICAM-1 in the model group increased significantly (P<0.05). When compared with the control group, they were markedly higher than those in the mangiferin group (P<0.05), suggesting that mangiferin can relieve inflammation injury in the brain tissue of hypertensive rats and that its mechanism may be related to the inhibition of MCP-1/CCR2 signaling pathway.
To further investigate the mechanism of mangiferin for antiinflammatory injury in hypertensive rats, we investigate the effect of mangiferin on the MCP-1/CCR2 signaling pathway in this study. MCP-1 is a representative of Monocyte Chemoattractant Protein (MCP) β subfamily, which has dual activity of chemotaxis and activating monocytes [13]. MCP-1 has powerful functions in inducing aggregation, wall attachment and migration of both monocyte and macrophage in vivo and in vitro. It causes the monocytes in the cycle to adhere to endothelial cells to promote the expression of adhesion molecules, participate in and expand inflammatory responses, and eventually form inflammatory cascades. The chemotaxis of MCP-1 is accomplished by its specific receptor CCR2, which plays an important role in MCP-l mediated signaling pathway [14]. MCP-1 is highly expressed in many inflammatory diseases such as hypertension, atherosclerosis, arthritis and cancer [15]. The results of Western blot assay showed that the expressions of MCP-1 and CCR2 in the model group increased significantly compared with the normal control group (P<0.05) and were markedly higher than those in the mangiferin group (P<0.05), proving that MCP-1/CCR2 signaling pathway plays an important role in the inflammatory damage of brain tissue in hypertensive rats and that its expression can be inhibited by mangiferin with the possible mechanism that mangiferin by inhibiting the expression of MCP/CCR2 signaling pathway, resists the activation of monocytes so as to inhibit the secreting activity factors of TTNF-α, IL-1β and ICAM-1 by the monocytes and then achieve the effect of inhibiting inflammation.
In this study the morphological changes of brain tissue in hypertensive rats were also observed and the result showed that: no obvious change was seen in the normal control group and in the model group there was local wedge type laceration with hemorrhage foci as well as the damages in polymorphic layer of cortex and subarachnoid hemorrhage on the surface of contused areas; in addition to visible cell necrosis, in some areas parts of nerve cell has smaller body as well as condensed cytoplasm and nucleus, showing morphological changes of apoptosis. Changes in the mangiferin group were similar with those in the model group but with lower degree of lesion. These results indicate that mangiferin can alleviate the brain damage in hypertensive rats.
In conclusion, Mangiferin can effectively reduce levels of inflammatory factors such as TNF-α, IL-1β and ICAM-1 in brain tissues of spontaneously hypertensive rats. It has obvious anti-inflammatory effect and protective effect on the brain injury with the mechanism possibly related to the inhibition of expression of MCP/CCR2 signaling pathway. The results of this study may provide valuable references for preventing and treating of hypertension by mangiferin.
References
- Meijles DN, Pagano PJ. Recent advances in hypertension: nox and inflammation in the vascular adventitia. Hypertension 2015; 67: 14-19.
- Saleh MA, Mcmaster WG, Wu J. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Investig 2015; 125: 1189-1202.
- Linz D, Hohl M, Schutze J. Progression of kidney injury and cardiac remodeling in obese spontaneously hypertensive rats: the role of renal sympathetic innervation. Am J Hypertens 2015; 28: 256-265.
- Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol 2015; 303: 359-367.
- Chen J, Chen Y, Hongyan LV. Effect of hyperbaric oxygen on lipid peroxidation and visual development in neonatal rats with hypoxia-ischemia brain damage. Biomed Rep 2016; 5: 136-140.
- Zhang S, Eitan E, Mattson MP. Early involvement of lysosome dysfunction in the degeneration of cerebral cortical neurons caused by the lipid peroxidation product 4-hydroxynonenal. J Neurochem 2017; 140: 941-951.
- Zoccali C, Leonardis D, Parlongo S. Urinary and plasma endothelin 1 in essential hypertension and in hypertension secondary to renoparenchymal disease. Nephrol Eur Dial Transpl Assoc Eur Renal Assoc 1995; 10: 1320-1323.
- Kameshima S, Okada M, Yamawaki H. Expression and localization of calmodulin-related proteins in brain, heart and kidney from spontaneously hypertensive rats. Biochem Biophys Res Commun 2016; 469: 654-658.
- Liu B, Hu B, Shao S. CD163/Hemoglobin oxygenase-1 pathway regulates inflammation in hematoma surrounding tissues after intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2015; 24: 2800-2809.
- Rivera DG, Balmaseda IH, Leon AA. Anti-allergic properties of Mangifera indica L. extract (vimang) and contribution of its glucosylxanthone mangiferin. J Pharm Pharmacol 2006; 58: 385-392.
- Garrido G, Gonzalez D, Lemus Y. Protective effects of a standard extract of Mangifera indica L. (vimang) against mouse ear edemas and its inhibition of eicosanoid production in J774 murine macrophage. Phytomed 2006; 13: 412-418.
- Rivera DG, Hernandez I, Merino N. Mangifera indica L. extract (vimang) and mangiferin reduce the airway inflammation and Th2 cytokines in murine model of allergic asthma. J Pharm Pharmacol 2011; 63: 1336-1345.
- Proost P, Wuyts A, Van D J. Human monocyte chemotactic proteins-2 and -3: structural and functional comparison with MCP-1. J Leuk Biol 1996; 59: 67-74.
- Chuang SY, Yang SH, Pang JHS. Cilostazol reduces MCP-1-induced chemotaxis and adhesion of THP-1 monocytes by inhibiting CCR2 gene expression. Biochem Biophys Res Commun 2011; 411: 402-408.
- Arakelyan A, Petrkova J, Hermanova Z. Serum levels of the MCP-1 chemokine in patients with ischemic stroke and myocardial infarction. Med Inflamm 2005; 2005: 175-179.