Research Article - Biomedical Research (2017) Volume 28, Issue 15
Effect of letrozole on the predicted adult height in boys with constitutional delay of growth and puberty: A clinical trial
Farzaneh Rohani1,2, Hosein Zaeri3,4*, Shirin Moarefian5, Sedighe Moradi6 and Mohammad Reza Alaii7
1Pediatric Endocrinologist Associated Professor of Iran University of Medical Sciences, Vice Chancellor of Pediatric Growth and Development Research Center, Iran University of Medical Sciences, Tehran, Iran
2Department of Pediatrics Endocrinology and Metabolism, Ali Asghar Children’s Hospital, Iran University of Medical Sciences, Tehran, Iran
3Pediatric Endocrinologist, Neonatal and Children’s Health Research Center, Golestan University of Medical Sciences, Gorgan, Iran
4Department of Pediatric Endocrinology and Metabolic Diseases, Taleghani Hospital, Gorgan, Iran
5Pediatrician, Firoozgar General Hospital, Iran University of Medical Sciences, Tehran, Iran
6Endocrinologist, Endocrine Research Center, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences, Tehran, Iran
7Pediatric Endocrinologist, Department of Pediatric Endocrinology and Metabolic Diseases, Mofid Children Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- *Corresponding Author:
- Hosein Zaeri
Pediatric Endocrinologist
Neonatal and Children’s Health Research Center
Golestan University of Medical Sciences, Iran
Accepted on July 10, 2017
Abstract
Objectives: The aim of this was to investigate the effect of letrozole (an aromatase inhibitor) on the Predicted Adult Height (PAH) in boys with constitutional delay of growth and puberty.
Methods: In this clinical trial, the boys with constitutional delay of growth and puberty referred to Endocrine Research Center, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences. Tehran was studied. Patients were treated with letrozole (letrofome, Iran Hormone Manufacturing Co., Tehran, Iran) at a dose of 2.5 mg for one year and then discontinued for 6 months and patients were followed up.
Results: During one year taking letrozole, bone age was progressed from 12.49 ± 0.9 y to 13.18 ± 0.7 y and 13.64 ± 0.7 at 6 months after discontinuation. PAH at baseline was 172.5 ± 5.4 cm and after 12 and 18 months was 175.2 ± 4.3 and 174.9 ± 5.4 cm, respectively. PAH mean difference at baseline and 12th month was 2.8 cm (P=0.02), and at 12th and 18th months was -0.3 cm (P=0.70). In bone densitometry, Zscore of spine during treatment with letrozole and after discontinuation increased to 0.084 ± 0.3 and 0.083 ± 0.1, respectively, and the difference was not significant. Mineral density of the femoral neck in the treatment period, 0.04 decreased, but after discontinuation the treatment, 0.4 increased that change was not significant.
Conclusions: The study provides an indication for the treatment with letrozole (aromatase inhibitors) increase PAH with slowing the progression of bone age and letrozole has no adverse effects on bone metabolism and serum lipids.
Keywords
Aromatase inhibitors, Predicted adult height, Constitutional delay of growth and puberty, Short stature, Serum.
Introduction
The most common cause of short stature and sexual infantilism in the boy adolescent is constitutional delay of growth and puberty. This diagnosis constitutes a large proportion of the growth disorders seen by paediatric endocrinologists. The child with constitutional delay typically is characterized by growth deceleration during the first two years of life, followed by normal growth progression paralleling a lower percentile curve throughout the pre-pubertal years, until a catch-up growth or pubertal growth spurt occurs late in adolescence. The pattern of growth usually runs in the family and fathers frequently report a similar pattern of growth and delayed puberty [1]. Ultimately, children with Constitutional Delay of Growth and Puberty (CDGP) have a late growth spurt, consistent with their late puberty, and this brings their height into the normal adult range. Final height is often in the lower part of the parental target height range. The predicted adult height (Bayley- Pinneau method) is usually greater than that achieved, especially when the skeletal age is extremely delayed, but this is difficult to reliably anticipate [2].
The use of aromatase inhibitors in children and adolescents expanded after the important role of estrogen in skeletal maturation became evident in the mid-1990 after reports of two young adult men in their 20’s, one with an estrogen receptor defect and the other with an inactivating mutation of the aromatase gene. Both were described as having tall stature and unfused epiphyses despite adult pubertal development [3,4]. Aromatase inhibition would theoretically negate the growth plate maturation resulting from estrogens. Studies do show that skeletal maturation is slowed by the treatment with growth continuing, implying that final height will be increased. Based on literature patients with this condition need only observation and reassurance or a short course of sex steroids at pubertal age to stimulate growth and to induce puberty, but several studies have shown that such approach, these patients may not reach to the favourite final height according to their genetic predisposition or are often in the low range of predicted adult height based on parental heights [5-10]. This study was performed in continuation of previous studies to better understand the effects of aromatase inhibitors on predicted adult height in patients with constitutional delay of growth and puberty.
Materials and Methods
Study was conducted according to the guidelines for Good Clinical Practice and the Declaration of Helsinki. All subjects provided written informed consent. This study had primarily designed as randomized double blind controlled trial and the subjects were randomly divided into two groups of receiving placebo and receiving letrozol. At the beginning of the study, we found that because of the small number of patients with CDGP, it was not possible to divide the patients into two groups. Therefore, the next enrolling patients were treated with letrozol.
However, fourteen patients were included in the study. One of the patients was excluded due to receiving placebo. Also, unwillingness of another patient to receive letrozole for one year and adhering from the study protocol caused to excluding of that patient from the study. Finally, the data of 12 patients were analyzed (letrofome, Iran Hormone Manufacturing Co., Tehran, Iran) at a dose of 2.5 mg per day for one year. All patients were evaluated in terms of height, weight, pubertal stage, bone mineral density and bone age in first visit and after 6, 12 and 18 months after treatment. Height and weight of patients were measured with standard stadiometer and bascule respectively, and recorded at each visit. Puberty stage was determined based on physical examination and Tanner stages.
Bone age was determined according to left hand radiography and Greulich and Pyle method and Predicted Adult Height (PAH) was calculated according to bone age and Bayley- Pinneau method.
In this clinical trial, 14 boys who referred to Endocrine Research Center, Institute of Endocrinology and Metabolism (Iran University of Medical Sciences, Tehran), because of short stature, delayed puberty, or both, and were still in Tanner stage 1 (G1) at the age of 13.75 y or more, and in medical history, physical examination and routine laboratory tests, was not found definite cause for the delay of growth and puberty, were enrolled.
Dual Energy X-Ray Absorptiometry (DXA) was used to measure Bone Mineral Density (BMD) of lumbar spine and femoral bone by a Norland Exell bone Densitometer and special paediatric software. All laboratory tests including blood sugar, fasting insulin, triglyceride, cholesterol (LDL, HDL, Total), haemoglobin, haematocrit, white blood count, FSH, LH, testosterone, estradiol, ferritin, SGOT, SGPT, ALP and BMD were checked and compared at first visit and after 6, 12, 18 months after treatment.
Fourteen patients were included in the study. One of the patients was excluded due to receiving placebo. Also, unwillingness of another patient to receive letrozole for one year and adhering from the study protocol caused to excluding of that patient from the study. Finally, the collected data of 12 patients were analyzed by SPSS-18 statistical software and paired t-test.
Results
A total of 12 patients were studied for 18 months. The mean age of the patients at baseline was 15.39 ± 0.7 y. Mean growth velocity at 12th month was 7.1 ± 2.1 cm and at 18th month was 7.5 ± 2 cm (P=0.65). Mean serum IGF-I at baseline 256.2 ± 100 ng/mL and at 12th and 18th month was 258.2 ± 90 and 365.5 ± 104 ng/dL, respectively. Changes in weight, height, Body Mass Index (BMI) and pubertal stage at different times were shown in Table 1.
| Variable | Baseline | After 12 months | After 18 months |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Height (cm) | 147.8 ± 4.6 | 155.6 ± 5.4 | 159.1 ± 5.6 |
| Weight (kg) | 36.9 ± 6 | 45.0 ± 6.2 | 47.1 ± 7.2 |
| BMI (kg/m2) | 17.2 ± 2.5 | 18.6 ± 2.2 | 16.2 ± 1.2 |
| Pubertal stage (Tanner) | P3G2 | P4G4 | P4G4 |
Table 1. Comparison of demographic and anthropometric variables of patients in different times.
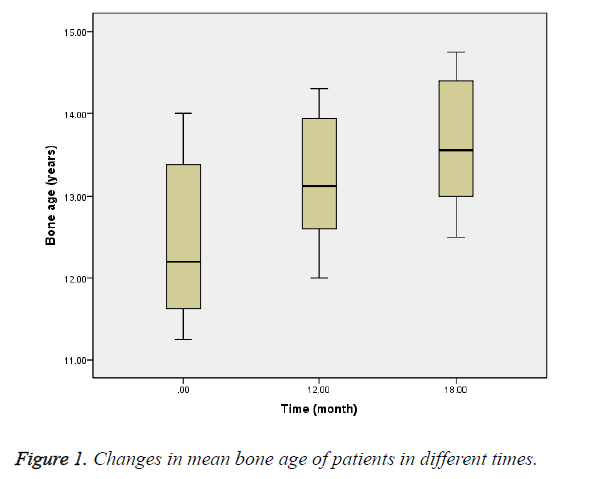
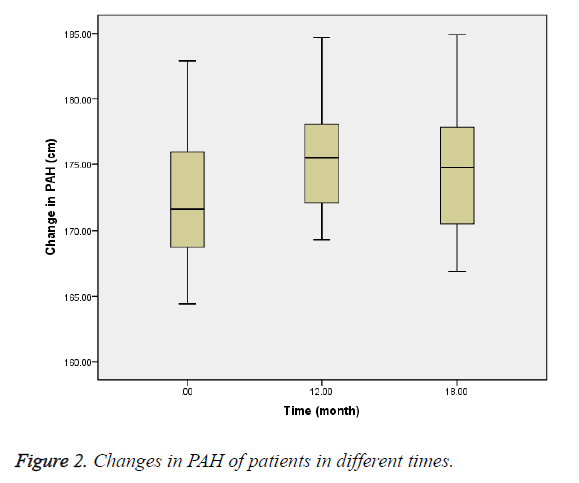
The mean bone age at baseline was 12.49 ± 0.9 y, and at 12th and 18th months were 13.18 ± 0.7 and 13.64 ± 0.7 y, respectively (Figure 1). PAH at baseline was 172.5 ± 5.4 cm, and at 12th and 18th months was 175.2 ± 4.3 and 174.9 ± 5.4 cm, respectively (Figure 2). PAH mean difference at baseline and 12th month was 2.8 cm (P=0.02), and at 12th and 18th months was -0.3 cm (P=0.70).
Testosterone serum concentration at baseline was 0.98 ± 0.95 ng/dL, and at 12th and 18th was increased to 5.3 ± 3.9 and 4.9 ± 2.7 ng/dL. Serum testosterone at baseline and 12th month was significantly different (P=0.002), but difference of serum testosterone at 12th and 18th months was not significant (P=0.754).
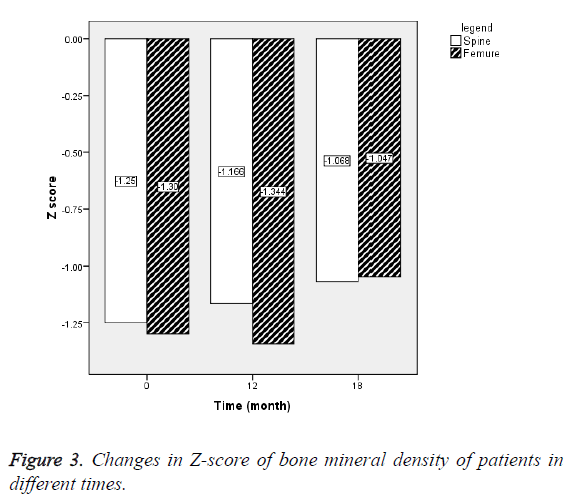
BMD of lumbar spine and femoral neck showed that Z-score of lumbar spine during treatment was increased with letrozole and after discontinuation. Z-score of femoral neck at 12th month was slightly reduced about -0.04, that increased to 0.3 after discontinuing treatment with letrozole (Figure 3). The changes in BMD mean of lumbar spine at baseline and 12th month (P=0.58) and 12th and 18th months (P=0.29) was not significant.
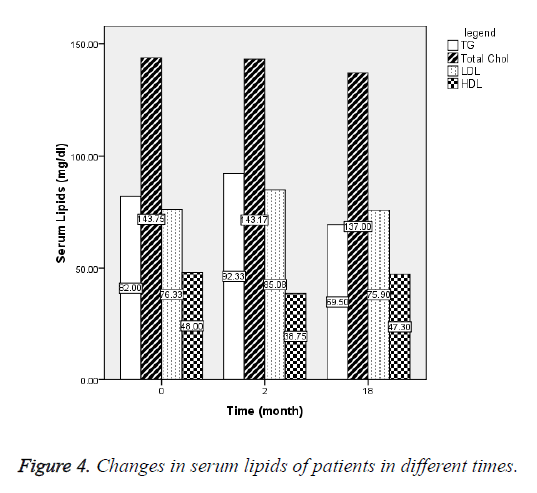
To study of the effects of letrozole on lipid metabolism, serum total cholesterol, HDL and LDL cholesterol, triglycerides and liver enzymes were measured at follow-up. Changes in these parameters that will result in discontinuation of therapy were not observed in any of the patients. Therefore, letrozole was well tolerated by patients and no complication was observed. Changes in serum concentration of lipids in different times are shown in Figure 4.
Discussion
One of the important issues in patients with constitutional delay of growth and puberty is to achieve a normal final height in a limited time with pubertal progression and closing growth plates. Treatment with androgens to accelerate the onset and progression of puberty had no effect on PAH. On the other hand, because the estrogen is an essential hormone for closure of the growth plates in boys, it can assume that decrease in estrogen levels thorough inhibiting the conversion of testosterone to estradiol by Aromatase Inhibitors (AI), delays bone age progression and increases final height.
In this study, the average growth rate during and after discontinuation of treatment with letrozole showed no significant difference (P=0.65), as the serum concentrations of IGF-I have increased from baseline to the months of 12 and 18.
The mean bone age was also 12.49 y that was lagging behind 2.9 y from the chronological age. Bone age progression was 0.69 y during treatment with letrozole which led to increasing PAH to 2.8 cm during treatment with letrozole compared with -0.3 cm after discontinuing it.
On the other hand the study results showed significant difference between the changes of PAH during treatment with letrozole and after discontinuing it. Krebs, et al. showed a significant increase in final height of a child with Idiopathic Short Stature (ISS) treated with aromatase inhibitors during a 5 y period.
In that patient, changes in final height and PAH were between 10.8 to 15 cm, and no permanent side effects due to medication were observed [11].
In study by Hero et al., 31 patients with ISS who randomly treated with letrozole and placebo for two years, PAH in the group receiving letrozole was 5.9 cm more than placebo recipients [8].
As in the study by Kumar et al., adding letrozole to treatment with growth hormone in a 13 y boy with short stature during one year caused a significant improvement of PAH without negative effects on puberty progression [12].
Krebs et al. showed a significant increase in final height of a child with Idiopathic Short Stature (ISS) treated with aromatase inhibitors during a 5 y period. In that patient, changes in final height and PAH were between 10.8 to 15 cm, and no permanent side effects due to medication were observed [11].
Mauras et al. in a study, 52 patients with growth hormone deficiency randomly treated with growth hormone-placebo and growth hormone-anastrozole (an aromatase inhibitor) for 36 months (50 patients for 12 months, 41 patients for 24 months and 28 patients for 36 months). The results showed that PAH of patients was 4.5 ± 1.2 and 6.7 ± 1.4 cm at 24 and 36 months after treatment with hormone-anastrozole and 1 cm in group treated with growth hormone-placebo [13].
Zhou et al. found similar results in their study. Letrozole significantly improves growth potential in a pubertal boy with growth hormone deficiency [14].
In randomized clinical trials by Dunkel et al., PAH of 23 patients with constitutional delay of growth and puberty were studied. In that study, patients were randomly treated with testosterone-placebo and letrozole-placebo for one year. Bone age progression in the group receiving letrozole during 18 months was 0.8 y slower in the group receiving placebo (0.9 y vs. 1.7 y). This delayed bone age progression in the group receiving letrozole led to increasing 5.1 ± 3.7 cm in PAH of these patients [1].
In Hero’s study about near final height, in 17 boys with constitutional delay of growth and puberty, who were randomly divided into two groups and treated with testosterone-placebo and testosterone-letrozole for one year, was reported an increase of height about 6.9 cm in the group treated with letrozole [15].
In the present study, bone age progression during treatment with letrozole was slower than after treatment discontinuation, and PAH increased 2.8 cm at 12 months after treatment, while after discontinuation of letrozole, PAH was decreased 0.3 cm. This increase of PAH in the present study is consistent with the results of Dunkel et al. studies in the patients with delay of puberty and Mauras et al. studies in the patients with growth hormone deficiency.
Effects of letrozole on bone mineral density: For short stature, as other uses of Aromatase Inhibitors (AI), their safety and side effects are important. One of the concerns about the use of aromatase inhibitors, their effects on BMD due to reduced estradiol concentrations. Therefore, BMD of lumbar spine, femoral neck and bone turnover markers such as alkaline phosphatase has been investigated in several studies. Studies by Dunkel et al. showed that bone density in both AI and control groups increase with puberty progression. In the present study, the Z-score of spine increased 0.084 ± 0.3 during treatment with letrozole and 0.083 ± 0.1 after discontinuing treatment, but the difference was not significant. Z-score of femoral neck did not change during treatment with letrozole, but after discontinuing treatment increased 0.4.
Effects of letrozole on serum lipids: Hero et al. was reported decreased HDL cholesterol and relative fat mass in boys treated with AI (letrozole) for 2 y. In Dunkel’s study, decreased HDL cholesterol in boys treated with AI (letrozole) was observed, but was not reported any changes in serum cholesterol and triglycerides with letrozole consumption [7]. In the present study, mean serum triglyceride concentration and LDL cholesterol during treatment increased 10 and 9 mg/dL, respectively, and after discontinuing treatment decreased 28 and 10 mg/dL, respectively, but these changes were not statistically significant. The mean serum HDL cholesterol concentration during treatment decreased 9.2 ± 13 mg/dL and after discontinuing treatment increased 8.5 ± 13 mg/dL, but these changes were not statistically significant.
In conclusion, the result of this study showed that treatment with letrozole (aromatase inhibitors) increase PAH with slowing the progression of bone age and letrozole have not any adverse effects on bone metabolism and serum lipids.
Acknowledgement
This clinical trial study was approved and supported by Iran University of Medical Science and Tehran University of Medical Science with grant number of 707. Authors wish to thank Iran Hormone Company that provide letrozole tablet (2.5 mg) for patients, free of charge. Also, authors would like to acknowledge colleagues in Institute of Endocrinology and Metabolism, Dr. Ghafoori and Ms. Mahmoodi, for their kind cooperation in conducting of the project. This project has registered in Iranian Registry of Clinical Trials (www.irct.ir) as IRCT 201112238496 N1. This trial has Iran University Ethical Committee approval.
Author Contributions
Farzaneh Rohani, Design, draft and writing; Hosein Zaeri, Analysis and draft; Shirin Moarefian, Interpretation, draft; Mohammad Reza Alaii, Design, analysis.
Conflict of Interest
None.
References
- Dunkel L, Wickman S. Novel treatment of short stature with aromatase inhibitors. J Steroid Biochem 2003; 86: 345-346.
- Wickman SI, Ankarberg-LC, Norjavaara E, Dunkel L. A specific aromatase inhibitor and potential increase in adult height in boys with delayed puberty: A randomized controlled trial. Lancet 2001; 375: 1743-1748.
- Damaiani D. Pharmacological management of children with short stature: The role of aromatase inhibitors. J Pediatr 2007; 83: 172-177.
- Shulman D, Diaz-Thomas A. Use of aromatase inhibitors in children and adolscents. What’s new? Curr Opin Pediatr 2010; 22: 501-507.
- Dunkel L, Wickman S. Novel treatment of delayed male puberty with aromatase inhibitors. Horm Res 2002; 57: 44-52.
- Dunkel L, Wickman S. Treatment of delayed male puberty: Efficacy of aromatase inhibition. J Pediatr Endocrinol Metab 2001; 14: 1541-1546.
- Dunkel L. Treatment with the aromatase inhibitor letrozole during adolescence increases near-final height in boys with constitutional delay of puberty. Clin Endocrinol 2006; 64: 510-513.
- Hero M, Norjavaara E, Dunkel L. Inhibition of estrogen biosynthesis with a potent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: a randomized controlled trial. J Clin Endocrinol Metab 2005; 90: 6396-6402.
- Dunkel L. Update on the role of aromatase inhibitors in growth disorders. Horm Res 2009; 71: 57-63.
- Dunkel L. Vertebral morphology in aromatase inhibitor-treated males with idiopathic short stature or constitutional delay of puberty. J Bone Miner Res 2010; 25: 1536-1543.
- Krebs A, Moske-Eick O, Doerfer J, Roemer-Pergher C, van der Werf-Grohmann N, Schwab KO. Marked increase of final height by long-term aromatase inhibition in a boy with idiopathic short stature . J Pediatr Endocrinol Metab 2012; 25: 581-585.
- Kumar KV, Jayaraman M, Verma A, Modi KD . Letrozole as a booster therapy in growth hormone deficiency. Indian Pediatr 2009; 46: 625-627.
- Mauras N. Anastrozole Increase predicted adult height of short adolescent males treated with growth hormone: A randomized, placebo-controlled, Multicenter trial for one to three years. J Clin Endocrinol Metab 2008; 93: 823-831.
- Zhou P, Shah B, Prasad K, David R. Letrozole significantly improves growth potential in a pubertal boy with growth hormone deficiency. Paediatrics 2005; 115: 245-248.
- Hero M. Treatment with aromatase inhibitor letrozole during adolescence increase near-final height in boys with constitutional delay of puberty. Clin Endocrinol 2006; 64: 510-513.