Research Article - Current Pediatric Research (2021) Volume 25, Issue 5
Effect of iron and zinc supplementation on linear growth of stunted Egyptian children under 5 years of age.
Michael F Fares*, Hanna Aboulghar, Sarah S ElTatawy, Miriam M Aziz
Department of Pediatrics, Cairo University, Cairo, Egypt
- Corresponding Author:
- Michael F Fares
Department of Pediatrics
Cairo University
Cairo
Egypt
Tel: 201006828040
E-mail: drmich82@gmail.com
Accepted date: May 6, 2021
Abstract
Background: Linear growth is the best indicator of children’s well-being and provides an accurate marker of inequalities in human development.
Aim: Study and compare the effect of micronutrients supplementation (Iron and zinc) on linear growth of infants and children less than 5 years of age suffering from stunted growth.
Patients and Methods: A randomized study included 60 children less than 5 years of age came to the department of clinical nutrition in a university hospital for nutritional assessment. Cases randomly divided equally into two groups. Group A received iron supplementation only and group B received supplementation with iron and zinc. Follow up of length/height was done after 6 months of supplementation.
Outcome Measures: Length/height for age and sex (birth to 5 years Z-scores, WHO, 2003).
Results: On comparing the length/ height of the two groups before (p=0.472) and after supplementation (p=0.923) respectively, no statistically significant differences were found between supplementation with iron only and with iron plus zinc. Yet, upon calculation the rate of change in linear growth after 6 months, a significant difference (p=0.002) was evident on comparing group B who received zinc plus iron (mean=13.6 cm increase in height) vs. Group A who only received iron (mean=10.8 cm increase in height).
Conclusion: Zinc supplementation in addition to iron led to significant acceleration of linear growth of stunted children compared to supplementation with iron alone.
Keywords
Stunting, Supplementation, Iron, Zinc, WHO Z-score.
Introduction
Improper nutrition in the first 1,000 days of a human’s life can lead to stunted growth, which is irreversible and accompanied by impaired cognitive ability and reduced school achievement [1]. About 50 percent of all children suffering from stunting lived in Asia and more than one-third in Africa [2].
Micronutrients play a major role in learning, cognitive functions, immune responses and cellular signaling. A study done in USA included children up to 18 years and found evidence that zinc supplementation had medium effect in improving height among children, especially who were young age and stunted before supplementation [3].
Iron has vital functions like acting as a co-factor for enzymatic reactions and carrying of oxygen to the tissues from the lungs. Iron deficiency worsen oxygen dependent cellular energy metabolism consequently, leads to defective linear growth and impaired cognition [4].
Patients and Methods
A randomized study was performed on stunted Egyptian children less than 5 years of age attended for nutritional assessment. An informed consent was obtained from the parents of all enrolled infants and children.
The study included 60 children less than 5 years of age suffering from stunted growth in the period from 1/5/2017–1/5/2018. Stunting was identified by assessing a child’s length or height (recumbent length for children less than 2 years old using a ruler and standing height for children age 2 years or older using stadiometer) and put the measurements on WHO Z-scores.
Exclusion criteria
• Children over 5 years of age.
• Ex-Premature infants.
• Children with physical growth disabilities, mental retardation or chronic illness such as sickle cell disease, cystic fibrosis, or severe protein-energy malnutrition.
Sample size
Sample size was calculated based on the previous work by Mozaffari-Khosravi et al. and Petry et al. the expected difference of height for age between the two groups was 1 ± 1.2 [5,6]. Using power 80% and 5% significance level; 24 participants in each group were required. This number was increased to 30 to compensate for possible losses during follow up. Sample size calculation was achieved using. PS: Power and Sample Size Calculation software Version3.1.2 (Vanderbilt University, Nashville, Tennessee, USA).
Cases evaluation
All cases were collected from the clinical nutrition department in a university. Following admission and enrollment, full history taking including age, gender, and prenatal, dietary and developmental history were taken together with a thorough clinical examination. MyPlate and 24-hour dietary recall assessment were used for proper assessment of food diversity and quantity Initial Anthropometric measurements (using WHO Z scores) to assess height or length/age for boys and girls [7-10]. Stunting uses a Z-score cut-off point of <-2 SD to classify low height-for-age as moderate and <-3 SD to classify severe.
Iron and zinc supplementation
Recommendations for zinc intake was 10 mg/day in children up to 5 years of age which make severe zinc deficiency much less common in developed countries [11]. Iron was given in the dose of 10-12.5 mg elemental iron for children up to 2 years of age. Children aged 2-5 years received 30 mg elemental iron according to WHO guidelines [12].
Follow up data
Follow up sessions for all the included cases every 2 weeks to ensure that they were compliant with the supplements. Assessment of length/height was done after 6 months using WHO Z-scores under 5 years of age. Compared to percentiles, Z-scores have a number of advantages: they are calculated based on the distribution of the reference population and comparable across ages, sexes, and anthropometric measures [13].
Statistical data
Data management and analysis were performed using SPSSc Statistics version 24 (IBMc Corp., Armonk, NY, USA). Comparisons between groups with respect to numeric variables were done using the Student’s t-test for normally distributed variables and the Mann-Whitney nonparametric test for the non-normally distributed variables. Comparisons between categorical variables were done by the chi-square test or fisher's exact for small sample size. All p-values are two-sided. P<0.05 were considered significant.
Results
Out of 60 enrolled children, 43% of them lived in rural areas and 57% were from urban areas. Prenatal history revealed that 40% of the mothers in group A suffered from anemia during pregnancy compared to 30% in group B. Percentage of infants who were received exclusive breast fed in group A was 76.7% vs. 60.0% of group B. Over 23% of infants in group A were formula fedvs. 40% of group B as shown in (Table 1).
| Group A | Group B | |||
|---|---|---|---|---|
| Count n=30 | % | Count n=30 | % | |
|
Residency |
||||
|
Rural |
16 | 53.30% | 10 | 33.30% |
|
Urban |
14 | 46.70% | 20 | 66.70% |
|
Non educated parents |
18 | 60% | 17 | 56.60% |
|
Prenatal history mothers suffered from anemia |
12 | 40% | 9 | 30% |
|
Exclusive breast feeding |
23 | 76.70% | 18 | 60.00% |
|
Formula feeding |
7 | 23.30% | 12 | 40.00% |
|
GIT infection |
7 | 23.30% | 9 | 30.00% |
Table 1: Demographic data, feeding, prenatal and past history among the studied groups.
Regarding diversity of the dietary elements, (Table 2) showed that 78.6% of the weaned children in group A had improper diversity in their meals and 71.4% didn’t eat proper quantity of food they should take. In group B 86.2% of the weaned children had improper diversity in their meals and 72.4% didn’t eat proper quantity of food they should take.
| Diversity and quantity of food | Group A | Group B | |||
|---|---|---|---|---|---|
| Count n=28 | % | Count n=29 | % | ||
| Diversity | Proper | 6 | 21.40% | 4 | 13.80% |
| Improper | 22 | 78.60% | 25 | 86.20% | |
| Quantity | Proper | 8 | 28.60% | 8 | 27.60% |
| Improper | 20 | 71.40% | 21 | 72.40% | |
Table 2: Diversity and quantity of food among the studied groups.
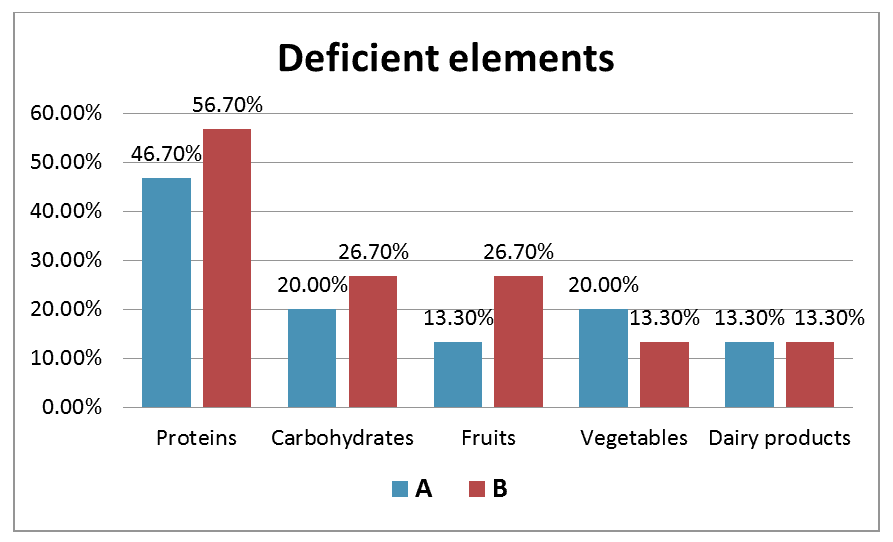
Proteins was the major deficient element inthe food of 46.7% of group A vs. 56.7% of group B as illustrated in (Figure 1). This was followed by lack of carbohydrates and vegetables (each 20%) then Fruits and Dairy products (each 13.3%) in the diet of group A. The other food elements deficient in group B were carbohydrates and fruits (each 26.7%) then vegetables and dairy products (each 13.3%).
Assessment of developmental milestones showed that 46.7% had delayed cognitive skills in group A compared to 36.7% in group B. Delayed motor milestones were found in 33.3% of group A compared to 23.3% in group B as shown in (Table 3).
| Delayed developmental milestones | Group A | Group B | ||
|---|---|---|---|---|
| Count n=30 | % | Count n=30 | % | |
| Motor development | 10 | 33.30% | 7 | 23.30% |
| Cognitive skills | 14 | 46.70% | 11 | 36.70% |
| Speech | 5 | 16.70% | 6 | 20.00% |
| Social development | 10 | 33.30% | 6 | 20.00% |
Table 3: Delayed developmental milestones among the studied groups.
Correlation of the gender to height/length in the initial visit revealed no statistically significant difference (Table 4). Median age of included cases was 13 months and there was no bias regarding age between the two groups (p=0.6). Also, on comparing length/height of both studied groups together there was no statistically significant difference between them in the first visit so no bias regarding to the initial length of children (p=0.472) as seen in (Table 5). Upon calculation the rate of change in length/height, a significant difference was found between the two groups on follow up after 6 months (p=0.002).
| Groups | Gender | P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ||||||||||||
| N | Mean | Standard deviation | Median | Minimum | Maximum | N | Mean | Standard deviation | Median | Minimum | Maximum | ||
| A | 19 | 71 | 8.4 | 68.5 | 59 | 90 | 11 | 70 | 9.2 | 67 | 59 | 90 | 0.641 |
| B | 8 | 70 | 6.5 | 70 | 61 | 81 | 22 | 69 | 8.8 | 66 | 59 | 97.5 | 0.344 |
Table 4: Correlation of gender to height/length on initial visit. p-value<0.05 is considered significant.
| Length/Height | Group A | Group B | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Median | Minimum | Maximum | Mean | Standard deviation | Median | Minimum | Maximum | ||
| Age months | 17.1 | 11.6 | 13.5 | 6 | 51 | 15.2 | 10 | 13 | 5 | 56 | 0.602 |
| On the first visit | 70.6 | 8.6 | 68 | 59 | 90 | 69 | 8.2 | 67 | 59 | 97.5 | 0.472 |
| Rate of Change (in cms) | 10.8 | 2.8 | 11.1 | 6 | 15 | 13.6 | 3.7 | 13.6 | 5.6 | 19.4 | 0.002* |
Table 5: Age initial length/height and rate of change between the two groups. p-value<0.05 is considered significant.
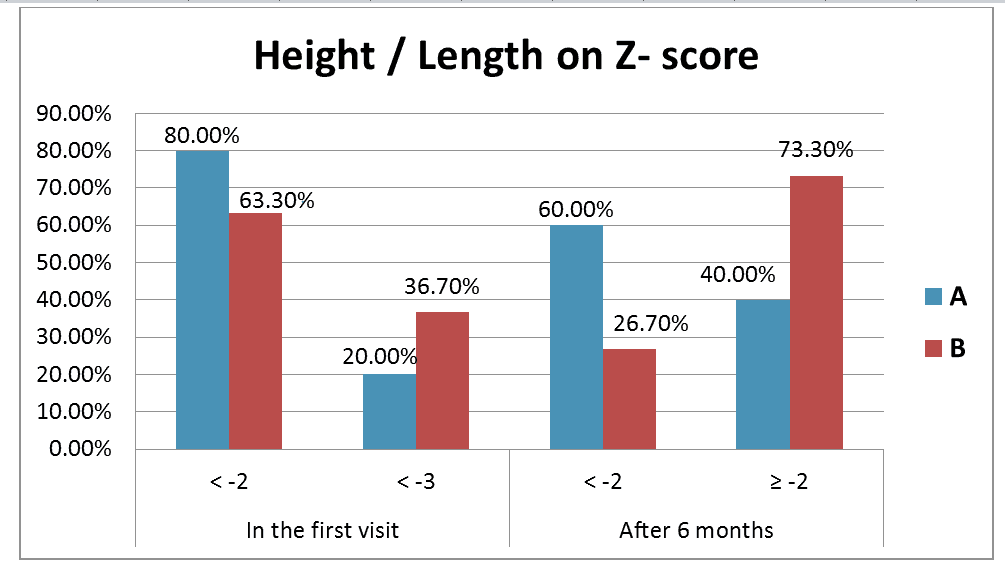
After 6 months of micronutrient supplementation, evaluation of each group separately showed that there was a statistically significant increase in length/height of each group (P-value<0.001) (Table 6). Meanwhile, (Figure 2) demonstrated that 60.0% of cases in group A became <-2 on WHO Z-score vs. 26.7% in group B and 40.0% in group A became =-2 compared to 73.3% in group B. Comparing of both groups together showed that the length/height of group B which received supplementation with iron plus zinc was significantly increased compared to group A which received supplementation with iron only (p-value=0.009).
| Length/height | Group A | Group B | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Median | Minimum | Maximum | Mean | Standard deviation | Median | Minimum | Maximum | |
| On the first visit | 70.6 | 8.6 | 68 | 59 | 90 | 69 | 8.2 | 67 | 59 | 97.5 |
| After 6 months | 78 | 7.5 | 75 | 68 | 95 | 78.2 | 7.1 | 76 | 70 | 103 |
| P-value | <0.001* | <0.001* | ||||||||
Table 6: The difference in length/height in each group separately after 6 months of supplementation. p-value<0.05 is considered significant.
Discussion
The aim of this study was to systematically evaluate the effect of iron and zinc supplementation on linear growth of infants and children below 5 years of age. The study was a randomized study including 60 children less than 5 years of age. They were randomly divided into two groups. Group A received iron only and group B received iron and zinc. Follow up of length/height was done after 6 months of supplementation.
As for place of residence, Shortage of economic resources means that food insecurity is more common among rural inhabitants [14]. Teenage pregnancy and high parity were prevalent in rural areas which cause nutrients depletion from the mothers because of physiological immaturity and the stresses of multiple pregnancies with short intervals in between [15].
About 60% of parents of the children included in our study were not educated. A study done by Vollmer et al. [16] in 2017 on childhood malnutrition in low-income countries reported a higher percentage of stunted children have non educated parents.
Many of children in our study received treatment for parasitic infestations. Many studies showed that parasitic infestation is common and has adverse outcomes on child growth and development. There is a increasing appreciation of the burden of helminths in pre-school children in whom infection may have the strong impact on child health [17].
About 50% of mothers in our study suffered from anemia during pregnancy. An intervention study examined the effect of nutrition on improving maternal and infant health in developing communities during pregnancy reported that African countries showed higher prevalence of maternal overweight, associated with poor quality of food and micronutrients deficiencies in women suffered from chronic malnutrition [18].
Delay in motor milestones and a cognitive skill among our studied groups was about 30%-40% in patients of each group while delay in speech and social development was about 20%-30%. This came in line with a study done in Nigeria by Jimoh et al. in which reported a prevalence of developmental delay of 35.4% among the study population [19]. Lower figures were reported among 9 to 24 months old American children Rosenberg et al. [20]. The cause for this variance may be related to several factors such as age range, number of developmental varieties assessed, method of assessment, wealth of countries and socioeconomic state.
In our study, 68% of children were Exclusively Breast Fed (EBF) and 32% received formula feeding in addition to breast feeding. A study done in Indonesia found that most of the subjects were breastfed for two years. This study reported that prolonged breast feeding duration was associated with increasing incidence of stunting [21].
About 30% of infants involved in this study were fed on cow’s milk. A study done by Zieglershowed that the feeding of cow’s milk has adverse effects on iron nutrition in infants [22]. Mostly due to low iron level in cow’s milk, occult intestinal blood loss and also high amounts of calcium and casein present in cow’s milk inhibit the absorption of dietary non heme iron.
Lack of diversity in the food was noticed in about 80% of children integrated in this study and about 70% of them didn’t receive quantity of food appropriate for their age. This was consistent with the results of a study conducted in Nepal [23]. This study found that a more diverse diet is beneficial for child growth. Another study conducted in Ethiopia mentioned that meals number and food diversity the child ate per day significantly affect the prevalence of stunting [24].
Our study showed that the element most deficient in the diet of stunted children was protein. A study done in rural southern Malawi by Semba et al. found that stunted children had lower serum concentrations of all essential amino acids compared with normal children [25]. In addition, stunted children had significantly lower serum concentrations of conditionally essential amino acids (arginine, glycine, and glutamine), non-essential amino acids and six different sphingolipids. If amino acids are not sufficient to synthesize proteins, growth regulatory pathways (mTORC1 and GCN2) will not allow growth to proceed [26].
We didn’t report significant difference in linear growth according to gender in our country. However, in India a study done by Jawaregowda and Angadifound that gender inequalities in food quality and quantity may contribute to malnutrition especially in settings where female off-springs are still considered less important compared to males [27].
According to Height for Age Z-score (HAZ) in our study, 80.0% of group A were placed on <-2 versus 63.3% of group B and 20.0% of group A were placed on <-3 versus 36.7% of group B. The rate of change after 6 months was 10.8 ± 2.8 in group A versus 13.6 ± 3.7 in group B. In a study done in Kenya by Ndiku et al. The percentage of children suffered from severe stunting (HAZ>-3) was 20.5% and this similar to the results of our study [28].
On comparing the length/height of the two groups before supplementation, no statistically significant differences were found between the two groups (p=0.472). After six months supplementation with micronutrients, the length/height of stunted children in each groups respectively was significantly improved (p<0.001).
Upon calculation the rate of change in linear growth after 6 months, a significant difference (p=0.002) was evident on comparing group B who received zinc plus iron (mean=13.6 cm increase in height) versus group A who only received iron (mean=10.8 cm increase in height). This statistically significant difference was also evident by plotting the length/height of the two groups on Z-score, as 73% of group B rose above the -2 value on Z-score compared to only 40% of group A (p=0.009).
These results were in agreement with a meta-analysis done by Imdad et al. which found that zinc supplementation is associated with a net benefit on linear growth. Results from a study by Fahmida et al. were in concordance with our results. This study showed that the benefit of the zinc plus iron supplementations were significantly higher among initially stunted cases [29,30].
The type of zinc supplementation used in our study was zinc sulfate and this is in agreement with most studies. In this study we gave zinc supplementation in a dose 10 mg/day. This came in line with a study by Dijkhuizen et al. which showed that preventive zinc supplementation in a dose of 10 mg/day has the most significant effect on linear length in children <5 years of age [31].
Conclusion
In our study iron supplementation alone without zinc also improved linear growth of stunted children. Our results are in line with a study conducted in Egypt by Ibrahim et al. which showed that iron is crucial for the growth of infants and preschool children in Egypt. In this study, a significant acceleration of subjects’ growth velocities were observed after iron supplementation for 6 months, associated with improvement in their height SDS.
Key Message
Assessment of anthropometric measures is an essential part in examination of infants and children to pick up any case of stunting early.
Dietary iron supplementation is recommended for exclusively breastfed term infants starting at four to six months of age. Exclusive breastfeeding after six months puts infants at risk for iron deficiency.
Regular dietary zinc intake is required because zinc cannot be produced or stored in the body.
Declaration of Competing Interest
All authors have approved the final article and have no conflict of interest to declare.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- UNICEF.https://data.unicef.org/topic/nutrition/malnutrition/#
- UNICEF/WHO/The World Bank. Levels and Trends in Child Malnutrition: UNICEF/WHO/The World Bank joint child malnutrition estimates, 2014.
- Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: A meta-analysis of randomized controlled trials. Am J ClinNutr 2002;75:1062–71.
- Ramesh J, Shaik MI, Srivalli J. Impact of Iron indices, mitochondrial oxidative capacity, oxidative stress and inflammatory markers on insulin resistance and secretion: A pathophysiologic perspective. J Diabetes Metab 2012;3:9.
- Mozaffari-Khosravi H, Shakiba M, Eftekhari MH, Fatehi F. Effects of zinc supplementation on physical growth in 2–5 years old children. Biological trace element research 2009;128:118-127.
- Petry N, Olofin I, Boy E, Donahue Angel, M Rohner F. The effect of low dose iron and zinc intake on child micronutrient status and development during the first 1000 days of life: a systematic review and meta-analysis. Nutrients 2016;8:773.
- Robert C. A New approach to dietary guidelines communications: Make MyPlate, your plate. Child Obes 2011;7: 349-351.
- Foster E, Bradley J. Methodological considerations and future insights for 24-hour dietary recall assessment in children. Nutr Res 2018;51:1-11
- https://www.who.int/tools/child-growth-standards/standards/length-height-for-age
- Iglesia I, Doets EL, Bel-Serrat S, Roman B, Hermoso M, et al. Physiological and public health basis for assessing micronutrient requirements in children and adolescents. The Eurreca network. Matern Child Nutr 2010; 6:84-99.
- https://apps.who.int/iris/bitstream/handle/10665/204712/9789241549523_eng.pdf.
- Preedy VR. Handbook of anthropometry: Physical measures 29 of human form in health and disease2012.
- Smith LC, Ruel MT, Ndiaye A. Why is child malnutrition lower in urban than rural areas? Evidence from 36 developing countries. International Food Policy Research Institute 2004;33:1285-305.
- Senbanjo IO, Olayiwola IO, Afolabi WA, Senbanjo OC. Maternal and child under-nutrition in rural and urban communities of Lagos state, Nigeria: The relationship and risk factors. BMC Res Notes 2013;6:286-96.
- Vollmer S, Bommer C, Krishna A, Harttgen K, Subramanian SV. The association of parental education with childhood undernutrition in low- and middle-income countries: comparing the role of paternal and maternal education. Int J Epidemiol 2017;46:312-23.
- Verani JR, Abudho B, Montgomery SP, Mwinzi PN, Shane HL, et al. Schistosomiasis among young children in Usoma, Kenya. Am J Trop Med Hyg 2011;84:787–91.
- Wrottesley SV, Lamper C, Pisa PT. Review of the importance of nutrition during the first 1000 days: maternal nutritional status and its associations with fetal growth and birth, neonatal and infant outcomes among African women. J Dev Orig Health Dis 2016;7:144–62.
- Jimoh AO, Anyiam JO, Yakubu AM. Relationship between child development and nutritional status of under-five Nigerian children. South African Journal of Clinical Nutrition 2018; 31:50–4.
- Rosenberg SA, Zhang D, Robinson C. Prevalence of developmental delays and participation in early child intervention services for young children. Pediatrics 2008;121:1503–9.
- Terati HY, Susanto E. Effects of diet and breastfeeding duration on the stunting status of children under 5 years of age at maternal and child health centers of the palembang regional office of health. Pak J Nutr 2018;17: 51-6.
- Ziegler EE. Adverse effects of cow's milk in infants. Nestle Nutr Workshop Ser Pediatr Program 2007;60:185-96.
- Busert LK, Neuman M, Rehfuess EA, Dulal S, et al. Dietary diversity is positively associated with deviation from expected height in rural Nepal. J Nutr 2016;146:1387–93.
- Motbainor A, Worku A, Kumie A. Stunting is associated with food diversity while wasting with food insecurity among under five children in east and west gojjam zones of amhara region, Ethiopia. PLoS ONE 2015;10(8): e0133542.
- Semba RD, Shardell M, SakrAshour FA, Moaddel R, Trehan I, et al. Child stunting is associated with low circulating essential amino acids. E Bio Medicine 2016;6:246-52.
- Ashorn P, Alho L, Ashorn U, Cheung YB, Dewey KG, et al. Supplementation of maternal diets during pregnancy and for 6 months postpartum and infant diets thereafter with small-quantity lipid-based nutrient supplements does not promote child growth by 18 months of age in rural Malawi: A randomized controlled trial. J Nutr 2015;145:1345-53.
- Jawaregowda SK, Angadi MM. Gender differences in nutritional status among under five children in rural areas of Bijapur district, Karnataka, India. Int J Community Med Public Health. 2015;2:506-9.
- Ndiku KM, Jaceldo-Siegl K, Singh P, Sabate J. Gender inequality in food intake and nutritional status of children under 5 years old in rural Eastern Kenya. Eur J Clin Nutr 2011;65:26–31
- Imdad A, Bhutta ZA. Effect of preventive zinc supplementation on linear growth in children under 5 years of age in developing countries: a meta-analysis of studies for input to the lives saved tool. BMC Public Health 2011;11 Suppl 3:S22.
- Fahmida U, Rumawas JS, Utomo B, Patmonodewo S, Schultink W. Zinc-iron, but not zinc-alone supplementation, increased linear growth of stunted infants with low haemoglobin. Asia Pac J Clin Nutr 2007;16:301–9.
- Dijkhuizen MA, Winichagoon P, Wieringa FT, Wasantwisut E, Utomo B, et al. Zinc supplementation improved length growth only in anemic infants in a multi-country trial of iron and zinc supplementation in South-East Asia. J Nutr 2008;138:1969-75.
- Ibrahim A, Atef A, Magdy RI, Farag MA. Iron therapy and anthropometry: A case-control study among iron deficient preschool children. Egyptian Pediatric Association Gazette 2017;65:95-100.