- Biomedical Research (2015) Volume 26, Issue 1
Effect of Hepatitis C Virus infection on the right ventricular functions, pulmonary artery pressure and pulmonary vascular resistance.
Canan Demir1, Mehmet Demir2*1Departments of Infectious Disease Bursa Şevket Yılmaz Education and Research Hospital, Bursa, Turkey
2Departments of Cardiology Bursa Yüksek İhtisas Education and Research Hospital, Bursa, Turkey
Accepted date: July 07 2014
Abstract
Infection with hepatitis C virus (HCV) is one of the most common causes of viral hepatitis worldwide. Multiple extrahepatic manifestations of HCV infection have been recognized. In this study we aimed to examine right ventricular systolic function and pulmonary artery pressure in HCV patients. The study included 50 HCV patients (mean age; 34±12 years) and 50 normal persons (mean age; 28±11 years) as control group. Transthoracic echocardiography was performed on all the participants. Right ventricular systolic parameters, pulmonary artery pressure and pulmonary vascular resistance (PVR) were compared between these two groups. In patients with HCV, the right ventricular fractional area change (RV FAC), tricuspid annular plane excursion (TAPSE) and RV myocardial systolic velocity (St) values were lower than the control group (31 ± 10 vs 48 ±12 %; 13.5 ± 1.5 vs 19.2 ± 3.4 mm and 8.3 ± 1.1 vs 17.7 ± 3.3 cm/s all p values<0.001;respectively); the right atrium (RA) and RV diameters were higher than controls (4.8 ± 1.3 vs 3.6 ± 0.6 cm, p<0.001; 4.4 ± 0.8 vs 3.3 ± 0.5cm p<0.001, respectively). Additionally systolic pulmonary artery pressure and PVR were higher than controls (36.3 ± 9.9 vs 23 ± 7.8 mmHg, 3.5 ± 1.1 vs 2.1± 0.8; p<0.001, respectively). Conclusion: The findings showed that HCV infection may be associated with right ventricular systolic dysfunction and pulmonary hypertension.
Keywords
Hepatitis C virus; cardiomyopathy; myocarditis; pulmonary artery pressure; pulmonary vascular resistance.
Introduction
The Hepatitis C virus (HCV) infection is a major public health problem worldwide. Additionally, HCV infection has been associated with extrahepatic involvements such as Sjögren’s syndrome, cryoglobulinemia, glomerulonephritis, lichen planus, and Hashimato’s thyroiditis [1,2]. It is also considered that there is a relation between HBV and HCV and oronary artery disease and heart failure [3-6].
Recent studies revealed that there is a relation between HCV and lung disease such as pulmonary fibrosis, chronic obstructive pulmonary disease and interstitial pneumonitis [7,8].
Several viruses, mainly parvovirus, adenovirus and enterovirus, may infect the myocardium. Since these agents cannot be found in many patients with myocarditis, other etiologic agents have been searched. Recently, the association of HCV infection with hypertrophic or dilated cardiomyopathy and myocarditis in patients has been recognised [9,10]. Moreover, we have established a relation between HCV infection and LV systolic and diastolic dysfunction and LV hypertrophy in our previous studies [6,11].
To our knowledge, so far there has been no study evaluating right ventricular systolic function and pulmonary hypertension in HCV patients. Our present study was conducted to explore any changes in systolic function of the right ventricle, pulmonary artery pressure and pulmonary vascular resistance (PVR) in HCV infected patients.
Materials and Methods
Selection of the patients
Fifty patients (with mean age 34±12 years) , who has been followed in the outpatient clinic of infection diseases department because of chronic hepatitis C (anti-HCV and HCV-RNA positive for at least 6 months), has normal liver enzymes and has not received antiviral treatment, were selected for the study.
The control group consisted of 50 persons, (with mean age 28±11 years ) who appealed to the cardiology and infectious disease outpatient clinic because of various reasons and did not have any identified structural cardiac pathologies. A physical examination, the medical history of patients, and the blood biochemistry were evaluated in all groups. The subjects were defined as hypertensive if their blood pressure was ≥140/90 mmHg or if they were receiving any antihypertensive medication. Diabetes mellitus was defined as the presence of a history of antidiabetic medication usage or fasting glucose level > 126 mg/dl. Smoking status was classified as smokers or those who never smoked.
Patients with coronary artery disease, heart failure, valvular disease, cardiomyopathy, hypertension, diabetes mellitus, chronic lung disease, sleep apnoea, thyroid dysfunction, anemia, malignancy, renal and hepatic insufficiency, chronic inflammatory disease, pregnancy, septicemia, cerebrovascular accident were exluded from the study. Intravenous drug abusers, alcohol drinkers, HIV and hepatitis B virus carriers were also excluded. All of the patients were in sinus rhythm and none of them were taking cardioactive medications like antiarrhythmics, antiplatelet, antipsycotics, and antihistaminics. Every patient signed an informed consent form and the local ethics committee approved the study.
Echocardiographic Measurements
Two-dimensional, M-mode, pulsed and colour flow Doppler echocardioagraphic examinations of all subjects were performed by the same examiner with a commercially available machine (Vivid 7 pro, GE, Horten, Norway, 2-4 mHz phased array transducer). During echocardiography, a one-lead electrocardiogram was used.
M-mode measurements were performed according to the criteria of American Society of Echocardiography (12,13). Left atrium (LA) diameter, LV end-sistolic and end-diastolic diameters were measured. LV ejection frection (EF) was estimated by Simpson’s rule.
Pulsed-wave mitral and tricuspid flow velocities were measured from the apical four-chamber view by inserting a sample volume to leaflet tips. Mitral and tricuspid early diastolic velocity (E, cm/sn), late diastolic velocity (A, cm/sn), E/A ratio, and E deceleration time (DT, ms) were determined. Each representative value was obtained from the average of three measurements. Doppler tissue imaging echocardiography was performed by transducer frequencies of 3.5-4.0 mHz, adjusting the spectral pulsed Doppler signal filter until a Nyquist limit of 15-20 cm/sn was reached, and using the minimal optimal gain. The monitor sweep speed was set at 50-100 mm/s to optimise the spectral display of myocardial velocities. LV myocardial peak systolic (s’, cm/s), early (e’) and late (a’) diastolic velocities and right ventricle (RV) myocardial peak systolic (St), early (RVe’) and late (RVa’) diastolic velocity, isovolumetric contraction time (ICT, ms), iso-volumetric relaxation time (IRT, ms), and ejection time (ET, ms) were obtained by placing a tissue Doppler sample volume in the basal segments of the lateral and septal walls both ventricul. Myocardial performance index (MPI) was calculated using (ICT+IRT)/ET formula for RV. By calculating the arithmetical mean value of the segmental values, mean RV St, e’,a’, mean MPI values were obtained. Therefore, Doppler tissue velocities given represent an average of the basal segments of the lateral septal walls. The tricuspid annular motion was recorded at the RV free wall for tricuspid annular plane excursion (TAPSE) and RV fractional area change (FAC) was measured from the apical four-chamber view according to the criteria of American Society of Echocardiography and European Associated Echocardiography [14]. PVR was calculated using (TR max velocity/RVOTVTİ) x 10 +0.16 [15].
Statistical Analyses
SPSS 16.0 statistical program (SPSS, Chicago, IL, USA) was used for statistical study. All values are given as mean ± standard deviation. Values between different groups were compared using the independent-samples t-test. A Chisquare test was used to assess differences between categorical variables. The relationship between parameters was determined using the Pearson coefficient of correlation. P-values <0.05 were considered significant.
Results
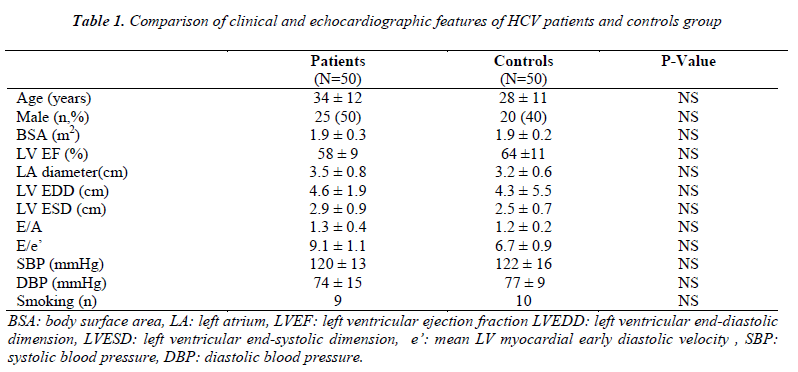
There was no statistically significant difference between the HCV group and controls with regard to age, gender, blood pressure, body surface area, smoking status, diameters of the left atrium and the left ventricle and left ventricular systolic and diastolic parameters (Table 1).
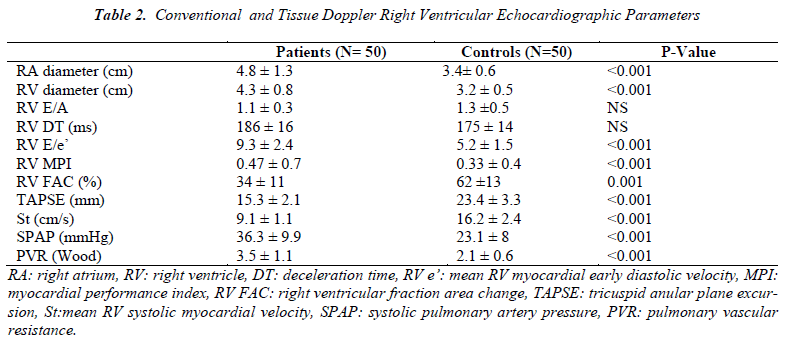
In HCV positive group, the RV FAC, TAPSE and St values were found to be lower (34 ± 10 vs 62 ±13 % and 15.3 ± 2.1 vs 23.4 ± 3.3 mm, and 9.1 ± 1.1 vs 16.2 ± 2.4 cm/s, all p<0.001, respectively); MPI, which shows both systolic and diastolic functions, was found to be higher in the patient group compared to the controls (0.47 ±0.7 vs 0.33 ± 0.2, p<0.001). The RA and RV diameters were found to be higher (4.8 ± 1.3 vs 3.4 ± 0.6cm and 4.3 ± 0.8 vs 3.2 ± 0.5cm both p<0.001, respectively); additionally systolic pulmonary artery pressure (SPAP) and PVR were found to be higher (36.3 ± 9.9 vs 23.1 ± 8 mmHg, and 3.5 ± 1.1 vs 2.1± 0.8; p<0.001, respectively). Also RV E/e’ ratio which shows diastolic dysfunction significantly higher in the HCV patients (9.3 ± 2.4 vs 5.2 ±1.5, p<0.001). No other statistically significant difference was found between the two groups with regard to the right ventricular diastolic parameters (Table 2).
Discussion
This study revealed that pulmonary systolic pressure and PVR were higher in HCV patients than in controls. Also there is a relationship between RV systolic dysfuction and HCV.
Recently, the importance of HCV infection in myocarditis and cardiomyopathy has been emphasised. HBV and HCV has been associated with atherosclerosis and HCV sero-positivity in patients with coronary artery disease and this was found to be related to cardiac failure and increased mortality[16]. Matsumori et al.[17] found anti- HCV positivity in 10.6% of the patients with hypertrophic cardiomyopathy and in 6.3% of the patients with dilated cardiomyopathy. Additionally, they found arrhythmia in 21.5% of anti-HCV positive patients. Hence, the authors suggested that HCV might play a role in several cardiac disorders with formerly unidentifiable etiology .
In our previous study, an association was also found between HCV infection and the left ventricular hypertrophy, in terms of the left ventricular systolic and diastolic dysfunction [6,11]. Wang et al.[18] found higher NT-proBNP levels, increasing with heart failure in the HBV/HCV patients not having liver failure, in comparison with the control group.
Recent studies revealed that there is a relation between HCV and lung disease such as pulmonary fibrosis, chronic obstructive pulmonary disease and interstisyal pneumonitis [7,8].
According to this situation, it is considered that HCV infections may increase heart failure and pulmonary dysfunctions. Despite a large number of studies done about the relation between cardiomyopathy and heart failure, the data about cardiac and pulmonar effects of HCV is limited.
In our study, we found lower RV FAC, TAPSE, St and higher MPI; which indicates RV systolic dysfunction in the patient group. Similarly a significantly high E/e’ ratio also indicated diastolic dysfunction in the HCV patients. In this study, we found a relationship between HCV infection and RV systolic and diastolic dysfunction. Also PASP and PVR were found higher than the control group. It may be considered that this situation causes RV systolic dysfunction, pulmonary dysfunction and portal hypertension due to hidden liver failure. Moreover, there may be some bioactive substances that we have not recognized yet, which may lead to obscure hepatic failure with normal AST/ALT and consequently are not metabolized in the liver and affect only RV, but not LV, since they are metabolized in the lungs; furthermore, hepatic failure may lead to portopulmonary hypertension and consequent RV dysfunction without manifesting clinical symptoms.
Limitations of the study
The most significant limitation of our study is the insufficient number of the patients. Other limitations include single transthoracic echocardiography assay, except physical examination other tests and evaluations were not performed for lung disease and sleep apnoea. Also for hepatic failure, other than AST and ALT further evaluation and imaging studies were not performed.
Conclusion
Our findings showed that HCV infection seems to be associated with the RV systolic dysfunction and pulmonary artery hypertension although the mechanisms of these are not known thoroughly. Therefore, cardiac involvement and pulmonary hypertension should be considered during the follow-up of a patient with HCV infection for extra hepatic involvement and these patients should be monitored with echocardiography. Further-more, HCV should be kept in mind for the patients who have cardiomyopathy, right cardiac failure and pulmonary hypertension with unidentifiable etiology. Further comprehensive studies may be needed to confirm our findings.
Conflict of interest
none declared
References
- Zignego AL, Brechot C. Extrahepatic manifestations of HCV infection: facts and controversies. J Hepatol 1999; 31: 369-76.
- Matsumori A. Symposium on clinical aspects in hepatitis virus infection. 5. Clinical practice of hepatitis: myocardial diseases, nephritis, and vasculitis associated with hepatitis virus. Intern Med 2001; 40:182-184.
- Amirzadegan A, Davoodi, Boroumand MA, Darabyan S,Dehkordi MR, Goodarzynejad H. Association between hepatitis B surface antibody seropositivity and coronary artery disease. Indian J Med Sci 2007; 61:648-655.
- Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group, Weber R, Sabin C, Reiss P, deWit S, Worm SW, Law M, Dabis F, D'Arminio Monforte A, Fontas E, et al. HBV or HCV coinfections and risk of myocardial infarction in HIV-infected individuals. Antivir Ther 2010; 15: 1077-1086.
- Demir M, Demir C. Effect of hepatitis B virus infection on right and left ventricular functions. Med Sci Monit 2012; 18: CR587-CR591.
- Demir M, Demir C. Effect of hepatitis C virus infection on the left ventricular systolic and diastolic functions. South Med J 2011 Aug; 104: 543-546.
- Aliannejad R, Ghanei M. Hepatitis C and pulmonary fibrosis: Hepatitis C and pulmonary fibrosis. Hepat Mon 2011; 11: 71-73.
- Kanazawa H, Hirata K, Yoshikawa J. Accelerated decline of lung function in COPD patients with chronic hepatitis C virus infection: a preliminary study based on small numbers of patients. Chest 2003; 123: 596-599.
- Matsumori A, Ohashi N, Sasayama S. Hepatitis C virus infection and hypertrophic cardiomyopathy. Ann Intern Med 1998; 129: 749-750.
- Matsumori A, Matoba Y, Sasayama S. Dilated cardiomyopathy associated with hepatitis C virus infection. Circulation 1995; 92: 2519-2525.
- Demir M, Demir C, Ãlçay A. Effect of Hepatitis C Virus Infection on the Left Ventricular Hypertrophy. Turkiye Klinikleri J Cardiovasc Sci 2009; 21: 315-319.
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N,Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989; 2: 358-367.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006; 7: 79-108.
- 14. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685-713.
- Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ: A simple method for noninvasive estimation of pulmonary vascular resistance. JACC 2003; 41:1021-1027.
- Matsumori A, Ohashi N, Hasegawa K, Sasayama S, Eto T, Imaizumi T, Izumi T, Kawamura K, Kawana M,Kimura A, et al. Hepatitis C virüs infection and heart diseases: a multicenter study in Japan. Jpn Circ J 1998; 62: 389-391.
- Matsumori A. Role of hepatitis C virus in cardiomyopathies. Ernst Schering Res Found Workshop 2006: 99-120.
- Wang L, Geng J, Li J, Li T, Matsumori A, Chang Y, Lu F, Zhuang H. The biomarker N-terminal pro-brain natriuretic peptide and liver diseases. Clin Invest Med. 2011; 1; 34: E30-E37.