- Biomedical Research (2014) Volume 25, Issue 1
Effect of flavonoids from Lotus (Nelumbo nuficera Gaertn) leaf on biochemical parameters related to oxidative stress induced by exhaustive swimming exercise of mice.
Hong-chao Xu1 and Mao-ye Wang2*1School of Physical Education, Shandong University of Finance and Economics, 250014 Jinan, China
2School of Physical Education, Shandong Normal University, 250014 Jinan, China
- *Corresponding Author:
- Mao-ye Wang
School of Physical Education
Shandong Normal University, 250014 Jinan
China
Accepted date: August 30 2013
Citation: Hong-chao Xu and Mao-ye Wang. Effect of flavonoids from Lotus (Nelumbo nuficera Gaertn) leaf on biochemical parameters related to oxidative stress induced by exhaustive swimming exercise of mice. Biomedical Research 2014; 25 (1): 1-6.
Abstract
The present study was undertaken to evaluate the effect of flavonoids from Lotus (Nelumbo nuficera Gaertn) leaf (FFL) on exhaustive swimming exercise-induced oxidant stress by measuring related biochemical parameters of mice. 32 male mice were randomly divided into four groups: control group, FLL low dose treated group, FLL middle dose treated group and FFL high dose treated group. The control group was given distilled water and the treated groups were given different doses of FLL (50, 100, 150 mg/kg) by gavage once a day for 28 days. 28 day later, mice were made to swim until being exhausted, then the exhaustive swimming time and some biochemical parameters related to oxidative stress were measured. The data showed that FFL can extend the exhaustive swimming time of the mice, as well as increasing the superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) activities, but decrease the malondialdehyde (MDA) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels. These results indicated that FLL possessed protective effects against exhaustive swimming exercise-induced oxidative stress.
Keywords
flavonoids from Lotus leaf; exhaustive swimming exercise; oxidant stress; mice
Introduction
The well-documented benefits of regular physical exercise include reduced risk of cardiovascular disease, cancer, osteoporosis, and diabetes [1]. Exercise can create an imbalance between oxidant and antioxidant levels, a situation known as oxidative stress [2,3]. Although regular physical exercise decreases oxidative stress, strenuous acute exhaustive exercise can cause oxidative stress and subsequent damage to cellular proteins, lipids and nucleic acids as well as changes to the glutathione system [4,5]. To avoid or minimize deleterious effect of exhaustive exercise- induced oxidant stress, there is an increasing evidence that antioxidant supplementation in humans and animals can reduce this oxidative stress [6–8].
Lotus (Nelumbo nucifera Gaertn) is a perennial aquatic crop with stout creeping yellowish white colored rhizomes [9]. Various parts of Lotus have been employed for medicinal proposes in traditional medicinal. A large number of pharmacological studies have demonstrated that Lotus leaf extract exhibit reducing blood pressure, antihyperlipidemic, antioxidant and hypoglycemic activities [10]. Flavonoids from Lotus leaf (FLL) is considered to be the principal active ingredients. FLL has been reported to reduce the formation of the lipid peroxidation and to be an effective antioxidant [11–13]. The effect of FLL supplementation on oxidative stress induced by exhaustive exercise is still poorly understood. The main purpose of this study was to evaluate the effect of flavonoids from Lotus leaf (FFL) on exhaustive swimming exerciseinduced oxidant stress by measuring related biochemical parameters of mice.
Materials and Methods
Materials and chemicals
Lotus leaves were collected in September-August in the northwest region of Shandong Province, China.
The plants were identified by Professor Yue Li, a biologist of Shandong Normal University (Jinan, China). A voucher specimen (registration number: 126951) has been deposited in herbarium of Shandong Normal University. Lotus leaves were dried in the air at 25-30 °C and then ground into powder. The kits including malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). DNA extractor kit was purchased from Wako Biochemicals (Osaka, Japan). 8-hydroxy-2′-deoxyguanosine (8-OHdG) enzyme-linked immunosorbent assay (ELISA) kit was purchased from Japan Institute for the Control of Aging (Fukuroi, Japan). All the other chemicals used were of analytical grade.
Preparation of flavonoids from Lotus leaf
Flavonoids from Lotus leaf (FLL) were prepared as described previously [14] with slight modifications. In brief, Lotus leaves powder (100 g) was suspended in 70% ethanol (ratio of solvent to raw material was 35) and were soaked for 4h, then under ultrasonication for 25 min in ultrasonic cleaner (KQ2200, Kunshan Ultrasonic Instrument Co., Jiangshu, China). A deep brown extract was obtained which was filtrated by using the filter (Shoa Shong, Shanghai, China). The filtrate was absorbed through D101 macroporous absorptive resin column (4.0 × 60 cm; Fubang Chemical Science Technologies Co., Tianjin, China) at the speed of 20 mL/min. The column was eluted with dH2O up to the washed liquid colourless and then eluted with 80% ethanol. The eluant was collected and evaporated by using a rotary evaporator (RE52AA, Yalong Biochemical Instrument Co., Shanghai, China) under reduced pressure at 40°C to get the FLL.
Selection of animals and care
Male Kunming mice (weighing 18 to 22 g) used in this study was purchased from the Laboratory Animal Center of Shandong Normal University (Jinan, China). The animals were maintained on a 12-hour light/dark cycle (lights on 07:00-19:00 hours) in a constant temperature (21-23 °C) and 50 ± 10% relative humidity colony room, with free access to food and water. The approval of this experiment was obtained from the Institutional Animal Ethics Committee of Shandong University of Finance and Economics (Jinan, China).
Exhaustive swimming exercise
After 1 week of acclimation, 32 mice were randomly divided into four groups equally based on body weight: control group (CG), FLL low dose treated group (FLG), FLL middle dose treated group (FMG) and FLL high dose treated group (FHG). The control group was given distilled water and the treated groups were given different doses of FLL (50, 100, 150 mg/kg) by gavage once a day for 28 days. 28 day later, mice were made to swim until being exhausted with wire of 5% body weight tied to their tails in the pool (length: 90 cm, width: 50 cm, depth: 50 cm) filled with 35 cm depth of water at 28-32 °C. Mice were regarded as being exhausted when they were underwater for 8 s [15,16], and their exhaustive swimming time was immediately recorded.
Analysis of biochemical parameters related to oxidative stress
All animals were anesthetized with ethyl ether and sacrificed immediately after the exhaustive swimming exercise. The gastrocnemius muscle of mice was collected and homogenized in buffer, and then they were centrifuged at 620 × g for 10 min at 4 °C. The samples were taken for biochemical estimations (SOD, GSH-Px and MDA). SOD, GSH-Px activities, and MDA levels were measured according to the recommended procedures provided by the commercial diagnostic kit.
DNA was isolated by the NaI extraction technique with the extractor kit. Then the isolated DNA was digested according to the previously published method [17]. The 8- OHdG levels of these samples were measured according to the recommended procedures provided by the commercial diagnostic kit.
Statistical analysis
All data were given as means ± standard deviation (SD). Comparisons between the means of various treatment groups were analyzed by using Dunnett’s t-test followed by analysis of variance (ANOVA). P < 0.05 was considered to be significant.
Results and Discussion
Effect of FLL on the exhaustive swimming time of mice
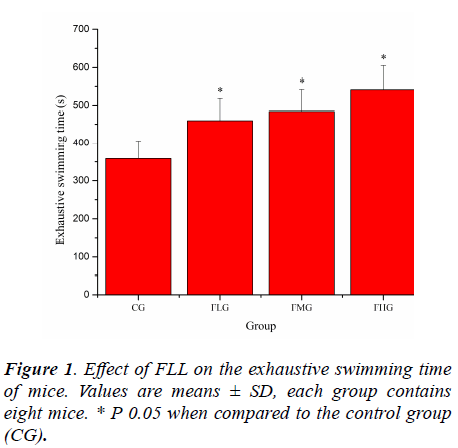
Forced swimming of animals has been employed as a criterion of their physical work capacity [18]. Previously study have pointed out that swimming has advantages over other forms of exercise, including the treadmill. Training is not required because rodents have a natural swimming ability and they are assumed to be highly motivated to avoid drowning when fatigue is imminent, assuring a high level of performance [19,20]. As shown in Figure 1, the exhaustive swimming time of mice in FLG, FMG and FHG were significantly prolonged compared with that in CG (P< 0.05), which was 1.28, 1.35 and 1.51 times longer that in CG, respectively. These results suggested that FLL could elevate the exercise tolerance of mice.
Effect of FLL on the MDA and 8-OHdG levels of mice
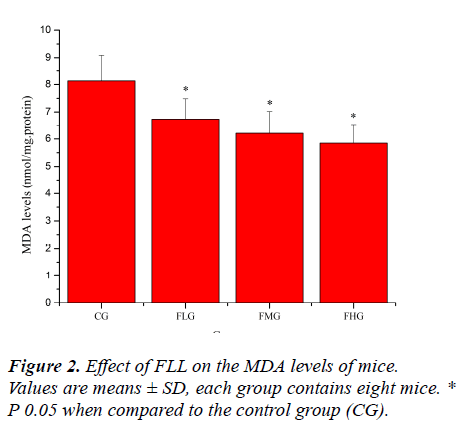
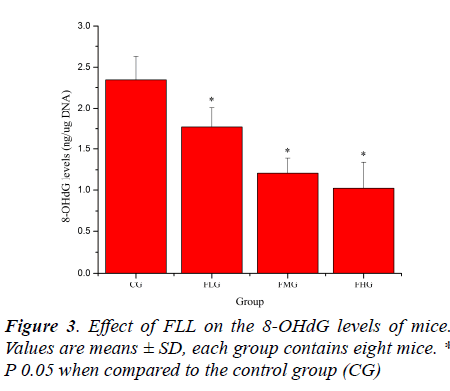
Oxidative stress induced by exhaustive exercise can significantly elevate markers of tissue per-oxidative damage because physical exercise promotes the production of ROS due to a substantial increase in oxygen consumption [21, 22]. The extent of oxidative damage is commonly measured through quantitative assessment of lipid peroxidation products such as MDA [23]. 8-OHdG, a DNA base-modified product generated by reactive oxygen species, is mutation prone and has also been shown to be a good marker for oxidative damage [24, 25]. As shown in Figure 2 and Figure 3, the MDA and 8-OHdG levels of mice in FLG, FMG and FHG were significantly decreased compared with that in CG (P< 0.05). These results indicated that FLL could reduce lipid and DNA per-oxidation, protected skeletal muscles from ROS-mediated oxidative damage after exhaustive exercise.
Effect of FLL on the activity of SOD and GSH-Px of mice
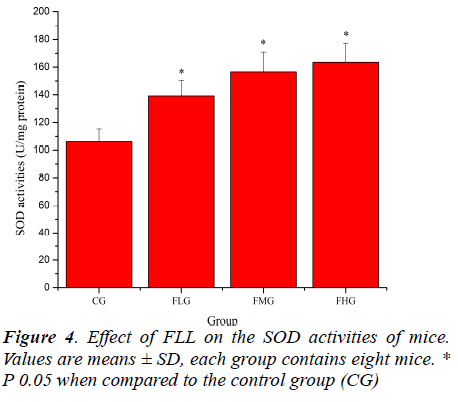
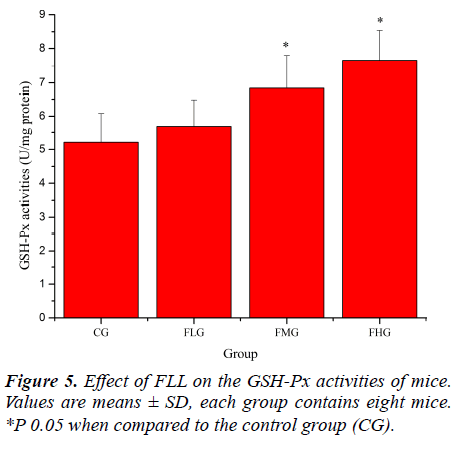
Antioxidant enzymes, which provide the primary defense against ROS generated during exercise, may be activated selectively during an acute bout of strenuous exercise depending on the oxidative stress imposed on the specific tissues as well as the intrinsic antioxidant defense capacity [26, 27]. SOD catalyses the conversion of the superoxide radical to H2O2 and H2 O; GSH-Px reduces H2 O2 to H2O by oxidizing glutathione (GSH). In addition, GSHPx can reduce lipid peroxides directly [28]. As shown in Figure 4, SOD activities of mice in FLG, FMG and FHG were significantly increased compared with that in CG (P< 0.05). As shown in Figure 5, GSH-Px activities of mice in FMG and FHG were significantly increased compared with that in CG (P< 0.05). Although GSH-Px activities of mice in FLG were also increased, no significant difference was observed (P > 0.05). These results suggested that FLL were able to up-regulate antioxidant enzyme activities to protect against exhaustive exerciseinduced oxidant stress.
In conclusion, the data showed that FFL can extend the exhaustive swimming time of the mice, as well as increasing the SOD, GSH-Px activities, but decrease the MDA and 8-OHdG levels. These effects might be due to its antioxidant property and showed that FFL acted as a good scavenger against free radical generation and thereby inhibits lipid and DNA per-oxidation. These findings indicated that FLL possessed protective effects against exhaustive swimming exercise-induced oxidative stress.
Acknowledgements
This work was supported by the Natural Science Foundation for Young Scientists of Shandong Normal University (Grant No. 2011147).
References
- Leeuwenburgh C, Heinecke JW. Oxidative stress and antioxidants in exercise. Curr Med Chem 2001; 8: 829-838.
- George BO, Osharechiren OI. Oxidative stress and antioxidant status in sportsmen two hours after strenuous exercise and in sedentary control subjects. Afr J Biotech 2009; 8: 480-483.
- Morillas-Ruiz JM, Villegas García JA, López FJ, Vidal-Guevara ML, Zafrilla P. Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clin Nutr 2006; 25: 444-453.
- Bloomer RJ, Goldfarb AH, Wideman L, McKenzie MJ, Consitt LA. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J Strength Cond Res 2005; 19: 276-285.
- Ciçek D. Exercise and oxidative stress. Anadolu Kardiyol Derg 2006; 6: 141-142.
- Ji LL. Antioxidants and oxidative stress in exercise.Proc Soc Exp Biol Med 1999; 222: 283-292.
- McCall MR, Frei B. Can antioxidant vitamins materially reduce oxidative damage in humans?. Free Radic Biol Med 1999; 26: 1034-1053.
- Sentürk UK, Gündüz F, Kuru O, Aktekin MR, Kipmen D, Yalçin O, Bor-Küçükatay M, Yeşilkaya A, Başkurt OK. Exercise-induced oxidative stress affects erythrocytes in sedentary rats but not exercise-trained rats. J Appl Physiol 2001; 91: 1999-2004.
- Yang D, Wang Q, Ke L, Jiang J, Ying T. Antioxidant activities of various extracts of lotus (Nelumbo nuficera Gaertn) rhizome. Asia Pac J Clin Nutr 2007; 1: 158-163.
- Wu MJ, Wang L, Weng CY, Yen JH. Antioxidant activity of methanol extract of the lotus leaf (Nelumbo nucifera Gertn.). Am J Chin Med 2003; 31: 687-698.
- Lin HY, Kuo YH, Lin YL, Chiang W. Antioxidative effect and active components from leaves of Lotus (Nelumbo nucifera ). J Agric Food Chem 2009; 57:6623-6629.
- Deng SG, Deng ZY, Huang L. Study on the antioxidative activity of flavonoids extracted from lotus leaves in-vitro. Food Sci Tech 2006; 31: 274-277.
- Jin SR, Yao LF, Yu ZP, Zhou MQ, Hu Z. Study on the extraction of flavonoids from lotus leaf and It's anti onxygenation. Anhui Agric Sci Bull 2006; 12: 95-98.
- Zhang L, Shan Y, Tang KJ, Putheti R. Ultrasound assisted xtraction flavonoids from Lotus (Nelumbo nuficera Gaertn) leaf and evaluation of its anti-fatigue activity. Int J Phys Sci 2009; 4: 412-422.
- Li FL, Xiao FR, Qi JS. Anti-fatigue effects of polysaccharides extracted from Rhodiolae Radix. Int Curr Pharmaceut J 2013; 2: 49-52.
- Hu QL, Zhang LJ, Li YN, Ding YJ, Li FL. Purification and anti-fatigue activity of flavonoids from corn silk.Int J Phys Sci 2010; 5: 321-326.
- Valls-Belles V, Torres Mdel C, Boix L, Muñiz P, Gonzalez-Sanjose ML, Codoñer-Franch P. Alpha-Tocopherol, MDA-HNE and 8-OHdG levels in liver and heart mitochondria of adriamycin-treated rats fed with alcohol-free beer. Toxicology 2008; 249: 97-101.
- Matsumoto K, Ishihara K, Tanaka K, Inoue K, Fushiki T. An adjustable-current swimming pool for the valuation of endurance capacity of mice. J Appl Physiol 1996; 81: 1843-1849.
- Akira W, Hiroyuki T, Futoshi O. Expression of vascular endothelial growth factor and its receptors in heart issue following short-term swimming training. Int J Sport Health Sci 2005; 3: 91-99.
- Zhang DR, Wan Y, Zu J, Zhan JG, Li L, Bai JQ.Morinda officinalis how enhances exercise endurance and possesses protective effects against oxidative stress of the rats after exercise. Afr J Microbiol Res 2010; 4: 1609-615.
- Liu DD, Chen C, Li RW. Protective effect of flavonoids from pericarpium citri reticulatae (chenpi) gainstoxidative stress induced by exhaustive exercise. Afr J Microbiol Res 2011; 5: 50-56.
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 2008; 88: 1243-1276.
- Requena JR, Fu MX, Ahmed MU, Jenkins AJ, Lyons TJ, Thorpe SR. Lipoxidation products as biomarkers of oxidative damage to proteins during lipid peroxidation reactions. Nephrol Dial Transplant 1996; 5: 48-53.
- Fukushima N, Kuromatsu R, Akiba J, Ando E, Takata A, Sumie S, Nakano M, Nakamura T, Kawahara A, Torimura T, Nakashima O, Okuda K, Yano H, Kage M, Kojiro M, Sata M. Characteristic expression pattern of oxidative stress in livers with cryptogenic hepatocellular carcinoma. Exp Ther Med 2010; 1: 809-816.
- Kang MH, Naito M, Tsujihara N, Osawa T. Sesamolin inhibits lipid peroxidation in rat liver and kidney. J Nutr 1998; 128: 1018-1022.
- Shan X, Zhou J, Ma T, Chai Q. Lycium barbarum Polysaccharides Reduce Exercise-Induced Oxidative Stress. Int J Mol Sci 2011; 12: 1081-1088.
- Thirumalai T, Therasa SV, Elumalai EK, David E. Intense and exhaustive exercise induce oxidative stress in skeletal muscle. Asian Pac J Trop Dis 2011; 1: 63-66.
- Powers SK, Ji LL, Leeuwenburgh C. Exercise traininginduced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc 1999; 31:987-997.