- Biomedical Research (2015) Volume 26, Issue 2
Effect of fast-acting asthma relieving mistura on serum LTC4 expression in mouse models of asthma.
1Pediatric Hospital, First Affiliated Hospital of Henan College of Traditional Chinese Medicine, No.19 Renmin Road, Jinshui Dist. 450000, Zhengzhou, China
2First People's Hospital of Zhengzhou City, No.56 East Street, Guancheng Huizu Dist. 450004, Zhengzhou, China
- Corresponding Author:
- Kun Zhao
Pediatric Hospital, First Affiliated Hospital of Henan College of Traditional Chinese Medicine Zhengzhou 450000, China
Accepted date: January 02, 2014
Abstract
This work aims to investigate the effect of traditional Chinese medicine fast-acting asthma relieving mistura (in Chinese, Suxiao Pingchuan Heji) on serum LTC4 expression in mouse models of asthma. 50 KM mice were randomly divided into Group A (control), group B (asthma model), group C (dexamethasone intervention), group D (fast-acting asthma relieving mistura intervention), and group E (intervention by dexamethasone together with fastacting asthma relieving mistura). The asthma models were established by intraperitoneal injection and aerosol inhalation with ovalbumin. The serum LTC4 level in mouse was determined by ELISA method, and the pathological change of lung tissue was observed underlight microscope. There was a little serum LTC4 expression in group A. The LTC4 level in group B was significantly higher than group A (P < 0.01), and that in group C, D, E was significantly lower than group B, respectively (P < 0.01). The inhibition effect on LTC4 expression in group E was obviously greater than group C and D, respectively (P < 0.01), with no significant difference between group C and D (P > 0.05). There was obvious change of inflammatory cell response in group B, with relatively mild inflammatory change in group C, D and E. No inflammatory change was found in group A. Over-expression of LTC4 is closely related to the occurrence and development of asthma. Fast-acting asthma relieving mistura can inhibit the over-expression of LTC4, thus preventing the further development of airway inflammation in asthma.
Keywords
Asthma, LTC4, fast-acting asthma relieving mistura, airway inflammation.
Introduction
Bronchial asthma (abbreviated asthma) is a chronic inflammatory airway disease involving many cells such as eosinophil, mastocyte, T lymphocyte, neutrophil, and airway epithelium [1]. As shown in many current studies, the pathogenesis of asthma is that, the inflammatory immune cells and secreted inflammatory mediators interact with various cytokines, leading to IgE-dependent immediate allergic reaction and eosinophil infiltration dominated chronic airway inflammation [2,3]. These inflammatory cells could synthesize and release a variety of inflammatory mediators. LTC4 is one of the primary inflammatory mediators in the occurrence and development of asthma [4,5]. However, the role of LTC4 in pathogenesis of inflammation in asthma, the relationship between LTC4 over-expression and the occurrence and development of asthma, and the effect of traditional Chinese medicine fast-acting asthma relieving mistura (FAARM) (in Chinese, Suxiao Pingchuan Heji) on LTC4 expression need to be further studied.
In this study, the role of LTC4 in pathogenesis of asthma and effect of FAARM on serum LTC4 expression in mouse models of asthma were investigated. The objective is to provide a theoretical basis for treatment of asthma and improve the life quality of patients with asthma.
Materials and Methods
Materials and apparatus
50 healthly male KM mice (20±3 g, 6-8 weeks of age, ordinary grade) were provided by Center of Henan province. FAARM produced by Jiangyin Tianjiang Pharmaceutical Co., Ltd. (China) was purchased from Chinese Dispensary in First Affiliated Hospital of Henan University. The main components included honey-fried herba ephedrae, raw radix paeoniae alba, asarum, morus alba, lepidium seed, white mustard seed, perilla-seed, fried radish seed, earthworm, coltsfoot and licorice. Dexamethasone (DEX) injection was provided by Shanghai Xupu Pharmaceutical Co., Ltd. (China). Ovalbumin (OVA) was provided by Sigma Company (USA). Aluminum hydroxide powder was purchased from Shanghai Shunqiang Biotechnology Co., Ltd. (China).
The main experimental apparatus were as follows: atomizing inhaler (Beijing Yadu Technology Co., Ltd., China), electrothermal constant temperature water bath box (20- 40°C, Beijing Medical Equipment Factory, China), adjustable liquid shifter (0.5-10, 20-50, and 50-100 μL), BRO-RAD 680 ELIASA instrument (Japan), PM-10AD optical microscopy (Olympus Co., Ltd., Japan), RM 2245 paraffin section machine and DM 5500B microscope (LEICA Company, German).
Animal grouping
50 KM mice were randomly divided into Group A (control), group B (asthma model), group C (DEX intervention), group D (FAARM intervention), and group E (intervention by DEX together with FAARM), 10 mice in each group.
Establishment of mouse models of asthma
The mouse models of asthma were established in the method of OVA sensitization and excitation. On the 1st day of experiment, all mice in group B, C, D, and E were intraperitoneally injected with 0.2 mL of fresh OVA solution (containing 100 ug of OVA and 2 mg of aluminum hydroxide). The injection was repeated l week later. On the 15th day, the mouse was placed in a transparent and airtight organic glass cabinet, and was treated by 30 minutes of aerosol inhalation with 2 mL of 2% OVA saline solution to excite the asthma. The treatment was performed once a day, for 2 consecutive weeks. The respiratory and systemic conditions of mice were observed. Appearance of symptom of faster breathing, sneezing and nose rubbing, perioral cyanosis, abdominal cramps, or nodding respiration indicates a successful excitation. For group A, equal amount of 0.9% saline instead of OVA was used for intraperitoneal injection and aerosol inhalation, using the molding method similar with other groups.
Drug intervention
The drug intervention in each group was conducted 30 minutes before excitation. Mice in group C were intraperitoneally injected with DEX (5mg/kg, equivalent to 0.5 mg/kg for human). In group D, mice were drenched with FAARM (concentration of crude drug, 1 g/mL; 15 g/kg), once per day for 14 successive days. In group E, FAARM was intragastricly administrated (15g/kg), and DEX was simultaneously intraperitoneally injected (5mg/kg). The treatment was conducted once per day for successive 14 days. The specific dosages of drugs were determined in the light of drug conversion formula of conventional animal to human with different body weight ("experimental animal and technology", edited by He S L) as follows: D2 = D1 × K2/K1 (D2, the calculated dosage; D1, the known dosage: K1 and K2, was obtained by table look-up).
Determination of LTC4 level and histopathological detection
Mice were anaesthetized by intraperitoneal injection of pentobarbital sodium. After eyeball removal, 1mL of blood was collected from each mouse. After centrifugation, the double antibody sandwich ELISA assay was performed for determination of serum LTC4 level to determine the level of LTC4 in serum
The right lung middle lobe tissue the middle lobe tissue of right lungwas separated from the mouse. After dehydration, being transparent, waxing, embedding, sectioning, mounting, parching, and HE staining, the general histopathological change of specimen was observed in light microscope, and the microscopic photography was conducted.
Statistical analysis
Data were represented by mean ± SD. Statistical analysis was performed by SPSS 17.0 statistical software by statistical software called SPSS 17.0. A t-test was used to analyze the difference between two groups, and single factor ANOVA was conducted for comparison among multiple groups. P<0.05, P<0.01 and P>0.05 were considered as statistically significant, highly statistically significant and not statistically significant, respectively.
Results
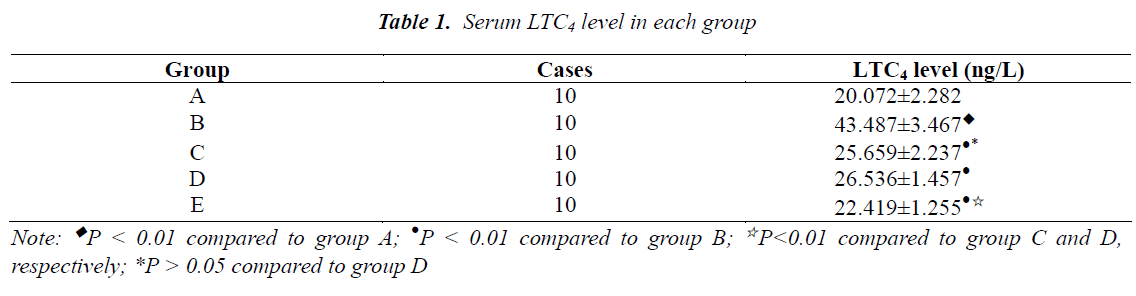
Serum LTC4 level in each group
As shown in Table 1, there was a little serum LTC4 expression in group A. The level of LTC4 in group B was significantly higher than group A (P<0.01), and that in group C, D and E was significantly lower than group B, respectively (P<0.01). The inhibitory effect on LTC4 expression in group E was obviously greater than group C and D, respectively (P<0.01), but the difference between group C and D was not statistically significant (P>0.05).
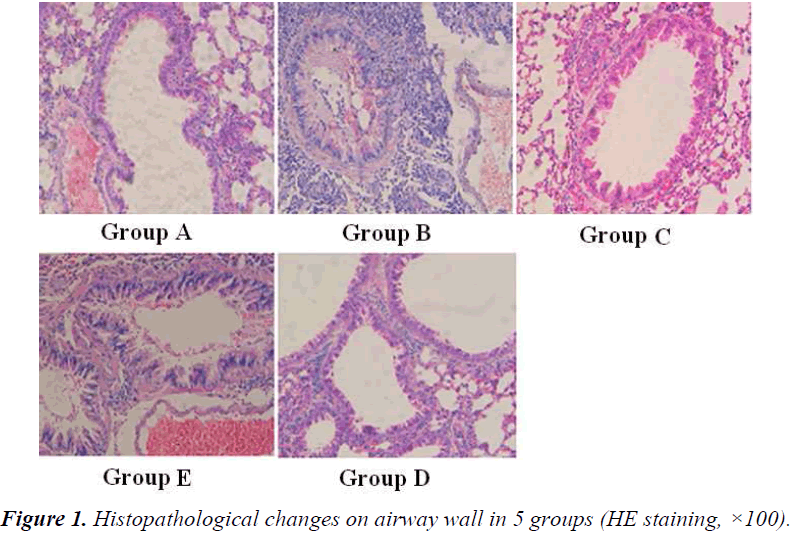
Histopathological change on airway wall in each group
Histopathological changes on airway wall in 5 groups were shown in Figure 1. In group B, there were obvious infiltration of eosinophils, lymphocytes and neutrophils, and duplicature on airway wall, with different degrees of epithelium necrosis and ablation necrosis and ablation in epithelium. In group E, the infiltration on airway wall was obviously reduced. No obvious inflammatory change was found in group A.
Discussion
Chronic airway inflammation is the basic pathological change of asthma [6]. In the process of inflammatory cell activation and infiltration,inflammatory mediator release and airway endothelium injury, the inflammatory cell gathering and migration to inflammation site is the most crucial step [7,8]. LTC4 is one of important inflammation factors in pathogenesis of asthma [9]. It can promote mitochysis of airway structural cell (e.g., bronchial smooth muscle cell), goblet cell hyperplasia, collagen deposition, and propria lamina fibrosis, thus facilitating the airway reconstruction for patients with chronic asthma [10]. Endothelin- 1 (ET-1) is the most powerful bronchial smooth muscle contraction agent. LTC4 can up-regulate the mRNA expression of ET-1 in airway epithelial cells and smooth muscle cells, and promote the release of ET-1 [11]. In addition, LTC4 can induce the P-selectin expression on surface of leukocytes and endothelial cells, leading to migration of PMN along blood vessel endothelium [12,13]. The over-expression of eosinophil specific Pselectin ligand can promote the adhesion of selective eosinophils with neutrophils [14]. This promotional effect on migration and aggregation of inflammatory cells can cause the production of LTC4, which makes more inflammatory cells enter the airway, so the inflammation was further aggravated [15,16]. Wang et al. [13] found that the LTC4 induced oxidative stress can aggravate the airway inflammation. Studies of Yin et al. [17] on children with asthma shows that, the serum LTC4 levels in children with mild, medium, and severe asthma increase accordingly, and were significantly higher than that in healthy children, respectively (P < 0.01). As found by Zhu et al. [18,19], the serum levels of LTC4 in patients with asthma in both attacking stage and relieving stage are higher than normal group. LTC4 is not only involved in allergic airway inflammation, but also an important factor in reconstruction of asthmatic airway.
In this study, the double antibody sandwich ELISA assay was performed to determine the serum LTC4 level, and the effect of FAARM on LTC4 expression was observed. Results indicated that, there was a little serum LTC4 expression in group A, and the LTC4 level in group B was significantly higher than group A. This indicated that, the overexpression of LTC4 played an important role in pathogenesis of inflammation in asthma. It could also be found in this study that, the LTC4 level in group C, D and E was significantly lower than group B, respectively. The inhibition effect on LTC4 expression in group E was obviously greater than group C and D, respectively, with no significant difference between group C and D. It was speculated that, the effect of FAARM on asthma might be partially related to its inhibition on over-expression of LTC4.
Histopathological detection results in this study showed that, in mice with asthma, there were obvious infiltration of eosinophils, lymphocytes,neutrophils and duplicature on airway wall, with different degrees of necrosis and ablation of epithelium. After intervention by FAARM, the infiltration of inflammatory cells on airway wall was significantly reduced. It was speculated that, FAARM could reduce the over-expression of LTC4 and inhibit the further development of inflammation in asthmatic airway, thus relieving the asthma attack.
It can be concluded that, the over-expression of serum LTC4 plays an important role in pathogenesis of asthmatic inflammation, and FAARM has remarkable inhibitory effect on expression of LTC4. There is no significant difference in intervention effect between FAARM and DEX. This indicates that, FAARM can be used to partially replace DEX in treatment of asthma, for reducing the side effects of hormonotherapy. However, the conclusions in this study still need to be further verified by repeated experiments and more clinical observation.
References
- Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004; 59: 469-470.
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleuk is a unique cytokine that stimulates both Th1 and Th2 responses depend ingon its cytokine milieu. Cytokine Growth Factor Rev 2001; 12: 53-72.
- Yang W, Kaur D, Okayama Y, Ito A, Wardlaw AJ, Brightling CE, Bradding P. Human lung mast cells adhere to human airway smooth muscle, in part, via tumor suppressor in lung cancer-1. J Immuno1 2006; 176: 1238-1243.
- El-Mezayen RE, Matsumoto T. In vitro responsiveness to LTC4 in combination by PBMC form patients with bronchial asmtha and atopicdermatitis. Clin Immunol 2004; 111: 61-68.
- Douwes J, Gibson P, Pekkanen J, Pearce N. Noneosinophilic asthma: importance and possible mechanisms. Thorax 2002; 57: 643-648.
- Hartwig C, Tschernig T, Mazzega M, Braun A, Neumann D. Endogenous LTC4 in experimentally induced asthma affects cytokine serum levels but is irrelevant for chinical symptom. Cytokine 2008; 42: 298-305.
- Ricciardolo L. Multiple roles of nitric oxide in the airways. Thorax 2003; 58: 175-182.
- G1NA. Global Strategy for Asthma Management and revention. www. ginasthma. org 2004.
- Leung TF, Tang NL, Lam CW, Li AM, Fung SL, Chan IH, Wong GW. RANTES G-401A polymorphism is associated with allergen sensitizeation and FEV1 in Chinese children. Respir Med 2005; 99: 216-219.
- Yoshimoto T, Tsutsui H, Tominaga K, Hoshino K, Okamura H, Akira S, Paul WE, Nakanishi K. LTC4, although antiallergic when administred with LTC4, stimulates LTC4 and histamine release by basophiles. Proc Ncad Sci USA 2009; 96: 13962-13966.
- Wild JS, Sigounas A, Sur N, Siddiqui MS, Alam R, Kurimoto M, Sur S. IFN-gamma-inducing factor (LTC4) increases allergic sensitization, serum IgE, cytokines, and airway eosinophilia in a mouse model of allergic asthma. J Immunol 2005; 164: 2701-2710.
- Kumano K, Nakao A, Nakajima H, Hayashi F, Kurimoto M, Okamura H, Saito Y, Iwamoto I. LTC4 enhances antigen-induced eosinophil recruitment into mouse airways. Am J Respir Crit Med 1999; 160: 873-878.
- Wang J, Mochizuki H, Todokoro M, Arakawa H, Morikawa A. Does leukotriene afect intracel lular glutathione redox state in cultured human airway epithelial cells? Antioxid Redox Signal 2008; 10: 821-828.
- Lukacs NW, Tekkanat KK, Berlin A, Hogaboam CM, Miller A, Evanoff H, Lincoln P, Maassab H. Respiratory syncytialvirus predisposes mice to augmented allergic airway responses via LTC4 mediated mechanisms. J Immunol 2001; 167: 1060-1065.
- Jeffrey J, Atkinson J, Robert M. Matrix metalloproteinase- 9 in lung remodeling. Am J Respir Cell Mol Biol 2003; 28: 12-24.
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998; 282: 2258- 2261.
- Peiling Yin, Rogers KH, Lewis DB. Beta2-microglobulin dependent T cells are dispensable for allergen induced T helper 2 responses. Exp Med 2006; 184: 1507-1512.
- Kraft M, Martin RJ, Wilson S, Djukanovic R, Holgate ST. Iymphocyte and eosinophl Influx Into alveolar tissue In nocturnel asthma. Am J Respire Crit Care Med 1999; 159: 228-234.
- Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999; 103: 779-788.