Research Article - Biomedical Research (2017) Health Science and Bio Convergence Technology: Edition-II
Effect of different chelating solutions on the push-out bond strength of various root canal sealers
H.Melike Bayram1*, Emre Bayram1, Merve Kanber1, Berkan Celikten2 and Feridun Saklar2
1Department of Endodontics, Faculty of Dentistry, Gaziosmapasa University, Tokat, Turkey
2Department of Endodontics, Faculty of Dentistry, Ankara University, Ankara, Turkey
- *Corresponding Author:
- Melike Bayram
Department of Endodontics
Faculty of Dentistry
Gaziosmanpasa University
Turkey
Accepted date: January 07, 2017
Abstract
Objective: The aim of this paper was to evaluate the effects of four final irrigation solutions, on bond strength to radicular dentin of three root canal sealers based on calcium silicate and resin.
Methods: This study used 96 extracted mandibular premolars. The teeth were sectioned transversally to obtain two sections. The resulting 192 samples were randomly divided into four irrigation groups: Group 1 was irrigated with 17% EDTA and 5.25% NaOCl; Group 2 with QMix; Group 3 with 0.2% chitosan solution; and Group 4 with distilled water. After these irrigation procedures, three specimens from each group were randomly chosen for SEM examination. Then, remaining teeth were randomly divided into three subgroups (n=15), according to the sealer used: AH Plus, MTA Fillapex, and Total Fill BC Sealer. A vertical load was applied using a universal testing machine. All statistical analyses were performed with the SPSS (ver. 20.0) software. The results were analysed with a One-Way Analysis of Variance (ANOVA) and a post-hoc Tukey’s test (p ≤ 0.05).
Results: AH Plus and Total Fill BC Sealer provided equal bond strengths to the root canal wall in all the groups except the distilled water group (p<0.05). MTA Fillapex showed lower bond strength values than those of either AH Plus or Total Fill BC (p>0.05). When chitosan was used, all root canal sealers showed their highest bond strength values.
Conclusions: Chitosan may serve as an alternative chelating agent for use with various root canal sealers.
Keywords
Chitosan, Bond strength, Root canal sealer, QMix
Introduction
The most important step in an endodontic treatment is to eliminate the micro-organism from the root canal system, which can be done by using the appropriate instruments and effective irrigants during the root canal treatment. However, due to the extremely complex anatomy of the root canal pulp space, these methods are not successful if employed alone [1]. Therefore, ideal endodontic irrigants must have additional properties, such as the ability to dissolve organic and inorganic tissues, antibacterial effects, and biocompatibility with the tissues [2].
Comb et al. [3] were the first to describe the smear layer, which occurs on the surface of the root canal wall after the root canal, is instrumented. Removal of the smear layer is an essential step in root canal treatment because the smear contains organic and inorganic remnants, such as odontoblastic projections, micro-organisms, and necrotic debris. In addition, it can block the dentinal tubules, hindering penetration of intra-canal antibacterial irrigants and root canal sealers [3]. To remove the smear layer during endodontic therapy, chelation agents, including Ethylenediaminetetraacetic Acid (EDTA), Qmix citric acid, and maleic acid, are used [4,5].
EDTA is widely used to remove the smear layer. However, due to its low antibacterial effect, it should be used in combination with sodium hypochlorite (NaOCl) or Chlorhexidine (CHX) during the root canal treatment. However, final irrigation with EDTA and NaOCl may cause erosion of the dentin [6-8]. In contrast, the use of EDTA and CHX in combination has shown excellent antibacterial effects and the ability to remove the smear layer, but this combination is known to generate a white precipitate [9]. The other irrigants used to remove the smear layer and disinfect is QMix, a two-in-one final irrigants that contains bisbiguanide antimicrobial agent (2% CHX), polyaminocarboxylic acid calcium-chelating agent (17% EDTA), and a surfactant. Qmix needs no additional mixing at chairside, it does not form a white precipitate, and it has less toxicity than 17% EDTA [5,10-12].
To improve the antimicrobial activity of the solution used for the final rinse without affecting the dentinal structure, new irrigation methods and solutions are continuously developed to eliminate the smear layer [13]. Chitosan can be used as a final irrigant; it is a natural polysaccharide, derived from the deacetylation of chitin, which is obtained from the shells of crabs and shrimp [14]. Chitosan has been studied for dental applications because of its nontoxicity, biocompatibility, bioadhesion, and biodegradability [15]. Kishen et al. [16] found that treating dentin with chitosan nanoparticles significantly reduced the number of Enterococcus faecalis cells. In addition, Silva et al. [17] found that chitosan effectively removed the smear layer from the dentin walls and caused less erosion than EDTA after mechanical preparation. Successful endodontic treatment depends on the total obturation of the complex root canal system with dimensionally inert, stable, biologically compatible root canal filling materials [18]. For this purpose, several new materials have recently been developed to improve the quality of sealants used in root canal treatments [19]. Their manufacturers claim that they effectively bond to intra-radicular dentin and gutta-percha or their cones. MTA Fillapex (Angelus; Londrina, PR, Brazil) is a recently developed calcium silicate-based (Csb) root canal sealer that is composed of Mineral Trioxide Aggregate (MTA), salicylate resin, natural resin, bismuth oxide, and silica nanoparticles. It does not have the negative characteristics of MTA, which include long setting time, difficult manipulation, and low flow capacity. MTA Fillapex simultaneously releases free calcium ions to accelerate the healing process by stimulating the regeneration of the adjacent tissues [20]. The other Csb root canal sealer and bioceramic root canal obturation system is Total Fill BC Sealer (FKG Dentaire SA; La Chaux de Fonds, Switzerland). It consists of calcium silicates, calcium hydroxide, calcium phosphate monobasic, and zirconium oxide. Its manufacturer claims that it is injectable, premixed, radiopaque, zero shrinkage, insoluble, and hydrophilic, meaning that it uses the moisture in the dentinal tubules to initiate and complete its setting reaction [21]. Irrigants may affect the adhesion of the filling materials to the root canal dentin. Adhesion is an important factor affecting long-term root canal treatment, because sealers that provide greater adhesion to the root canal dentin may also provide greater resistance to root fracture and exhibit less leakage [21]. The literature contains many studies of the anti-biofilm efficacy, bioactivity, chelating effects, and antibacterial effects of chitosan [16,22-24]. However, chitosan’s effects on the ability various root canal sealers to adhere to the dentin structure have not been investigated compared to the effects of QMix and EDTA irrigants. Therefore, the purpose of this study was to use the push-out test method to evaluate the effects of four final irrigants, EDTA, QMix, chitosan, and distilled water, on the adhesion to radicular dentin of root canal sealers based on calcium silicate (MTA Fillapex and Total Fill BC Sealer) and resin (AH Plus).
Materials and Methods
Sample selection
This study used 96 extracted, single-rooted, human mandibular premolars of similar size that had been stored in 0.5% chloramine-T until required. The teeth were carefully examined under an operating microscope (Zeiss; Oberkochen, Germany) and those with immature apices, caries, restorations, fractures, or cracks were excluded from the study. Preoperative radiographs were taken in the mesiodistal and buccolingual directions to confirm the presence of a single canal without previous root canal treatment, resorptions, or calcifications.
Sample preparation
To ensure standardization, teeth were partially removed from the coronal part to achieve a standard root length of 12 mm, and the middle third of the roots were sectioned transversally into two sections 2 mm thick ± 0.1 mm using a water-cooled, low-speed ISOMET diamond saw (Buehler; Lake Bluff, NY, USA). The thickness of each slice was measured with a digital calliper that had an accuracy of 0.001 mm (Avenger Products; North Plains, OR, USA). After inspection with an optical microscope (OPMI Pico; Zeiss Co.; Jena, Germany), specimens with round canals were selected for use, to standardize the configuration of the root canal orifice shape. In each section, the lumens of the root slices were prepared with post drills (GT® Fiber Posts and Drills; Dentsply Tulsa Dental Specialties; Tulsa, Oklahoma, USA) to obtain cavities of a standard 1mm in diameter.
Determining groups
All samples (n=192) were immersed in a solution of 5.25% sodium hypochlorite for 3 min, and then immediately washed in distilled water and dried. Then, samples were randomly divided into 4 main groups. The samples in Group 1 were immersed in a solution of 17% EDTA for 3 min followed by immersion in a solution of 5.25% NaOCl for the same period of time and then were dried with paper points. The samples in Group 2 were immersed in a solution of QMix for 60-90 s and then dried with paper points. For samples in Group 3, a 0.2% chitosan solution was prepared by diluting 0.2 g of 90% deacetylated chitosan (Ankara University, Department of Chemical Engineering) in 100 ml of 1% acetic acid with stirring for 2 h by a magnetic stirrer. Then, the samples were immersed for 3 min in a chitosan solution and the root canals dried with paper points. The samples in Group 4 were immersed for 3 min in distilled water only.
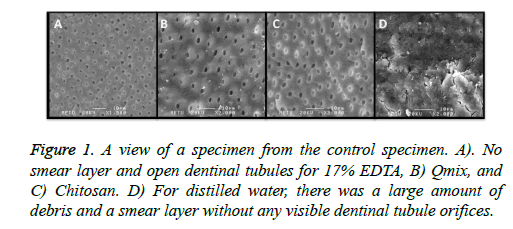
After these irrigation procedures, three specimens from each group were randomly chosen to have their root canal walls visualized under a Scanning Electron Microscope (SEM) (Figure 1) to observe the effectiveness of smear layer removal. Then, each group was randomly divided into 3 subgroups (n=15), according to the sealer used.
Groups 1a, 2a, 3a, and 4a were filled with AH Plus. Groups 1b, 2b, 3b, and 4b were filled with MTA Fillapex. Groups 1c, 2c, 3c, and 4c were filled with Total Fill BC Sealer. All root sealers were mixed and used according to the manufacturers’ instructions. A scalpel was used to remove excess materials from the surfaces of the specimens. Specimens were inspected using a microscope (10X), and those with irregularities such as defects, fractures, and gaps between dentin and the test material were discarded. Thereafter, the samples were stored at 37°C and 100% humidity for 7 days to ensure complete setting of the test materials.
Push-out testing
The filling material was loaded with a cylindrical plunger 0.85 mm in diameter to provide the most extended coverage over the filling material without touching the canal wall. The plunger was connected to the load cell of a universal testing machine (Instron Corp; Norwood, MA, USA). A vertical load was applied on the root canal filling through the coronal direction at a speed of 1 mm/min until a bond failure occurred. The bond strength at the failure was calculated in MPas by dividing the load in Newtons (N) by the area of the bonded interface.
After the push-out test, the samples were examined exhaustively to identify the modes of failure under a stereomicroscope (Olympus SZ61; Olympus Optical Co; Tokyo, Japan) at 25X magnification. The failures were classified according to Skidmore et al. [25] as type I (adhesive failure at the sealer-dentin interface), type II (cohesive failure within the sealer or dentin), or type III (mixed failure in both the sealer and dentin).
All results were analysed by one-way Analysis of Variance (ANOVA) and a post-hoc Tukey’s test to determine significant differences among the groups. IBM SPSS, ver. 20.0 software was used for all analyses. The significance level was set at p ≤ 0.05.
Results
Table 1 shows the push-out bond strength values in MPas. The AH Plus and Total Fill BC Sealer showed similar strengths in bonds to the root canal wall in all groups except the distilled water group (p<0.05). However, MTA Fillapex had lower bond-strength values than those of either AH Plus or Total Fill BC Sealer (p>0.05). When chitosan was used as a final irrigant, all root canal sealers presented their highest bond strength values, and all sealers showed their lowest bond strength values when distilled water was used. Final irrigation with 17% EDTA, Qmix, and chitosan improved the bond strength of root canal sealers to radicular dentin. Table 2 shows the percentage of failures mode for each group. Cohesive failure between the resin sealer and dentin was the most frequent type of failure in the AH Plus and Total Fill BC groups, while adhesive failure was the most frequent type in the MTA Fillapex group. After SEM examination, the presence of smear tissue was observed in the distilled water group, although no smear layer was detected in the EDTA, QMix, and chitosan irrigation groups (Figure 1).
| Material | n | Group 1 17% EDTA (mean ± SD, MPa) | Group 2 Qmix (mean ± SD, MPa) | Group 3 Chitosan (mean ± SD, MPa) | Group 4 Distilled water (mean ± SD, MPa) |
|---|---|---|---|---|---|
| AH Plus | 60 | 2.564 ± 0.778A,a | 2.554 ± 0.780A,a | 2.988 ± 0.553A,a | 0.605 ± 0.285A,b |
| MTA Fillapex | 60 | 0.384 ± 0.223B,a | 0.413 ± 0.231B,a | 0.861 ± 0.427B,b | 0.334 ± 0.238A,a |
| Total fill BC sealer | 60 | 2.159 ± 0.561A,a | 2.054 ± 0.854A,a | 3.333 ± 1.241A,b | 1.181 ± 0.334B,c |
| *Within the same column, the means with the same uppercase superscript letter are not statistically different (P>0.05), while, within the same row, the means with the same lowercase superscript letter are not statistically different (P>0.05). | |||||
Table 1: Push-out bond strength values (Mean ± SD) for the different groups.
| EDTA AH Plus | EDTA MTA Fillapex | EDTA total fill BC | Qmix AH Plus | Qmix MTA Fillapex | Qmix total fill BC | Chitosan AH Plus | Chitosan MTA Fillapex | Chitosan total fill BC | Distilled water AH Plus | Distilled water MTA Fillapex | Distilled water total Fill BC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adhesive failure | 27% | 40% | 7% | 33.30% | 40% | 20% | 20% | 27% | 13% | 13.30% | 53% | 13% |
| Cohesive failure | 33% | 27% | 53% | 33.30% | 27% | 40% | 60% | 27% | 47% | 73.30% | 20% | 67% |
| Mixed failure | 40% | 33% | 40% | 33.30% | 33% | 40% | 20% | 46% | 40% | 13.30% | 27% | 20% |
Table 2: Percentage of the failure modes for the each groups.
Discussion
Adhesion of root filling materials to dentin is essential to the success of endodontic treatments. Such adhesion is necessary to eliminate leakage and give the material resistance to displacement forces that occur during condensation of permanent restorative materials [26-28]. Therefore, evaluating the bond strengths of materials using mechanical testing can provide important information for clinical practice. Many techniques, such as push-out bond strength tests, tensile tests, and shear tests, can be used to survey the bond strength of materials to dentin. The present study used the push-out test to evaluate the adhesion of various root canal sealers because the push-out test is reportedly efficient, practical, and reliable [29-31].
A number of factors, including the presence or absence of smear layer, intermolecular surface energy of the dentin structure, surface tension of the sealers, and wetting capability, may affect adhesion properties [32]. The smear layer that forms during root canal preparation and the fact that it may inhibit the penetration of irrigation agents and sealers into the dentinal tubules [32]. For these reason, removal of the smear layer is recommended [33,34], and this study used the 17% EDTA, QMix, and chitosan solutions to remove it. SEM analysis of the samples in each group showed that the three solutions provided to remove the smear layer.
Many studies have investigated the effects of endodontic irrigants, smear layer, and various environments on the push-out bond strength of various root canal sealers [35-38]. However, the present study is the first to evaluate the effect of chitosan on the bond strength of various root canal sealers. In this study, all sealers showed their highest bond-strength values when canals were irrigated with 0.2% chitosan. According to Silva et al. [17], application of chitosan for 3 min is most effective for removing the smear layer and minimizing erosion of dentin surfaces. In light of this information, and due to the minimal erosion effects and chelating effects of chitosan, it may have increased the bond strength to the dentin of the sealers tested in this study.
The present study demonstrated that QMix and 17% EDTA had similar effects on the bond strength of all the sealers tested (p>0.05). QMix consists of EDTA, CHX and a surfactant, and it can remove the smear layer alone. Surfactants reduce surface tension and increase wettability and, as a result, enhance the flow rate of the irrigating solution, thus effectively removing the smear layer and increasing the ability of the sealer to penetrate the dentin [39]. In addition, the CHX in QMix provides a long-term antibacterial effect. QMix has been suggested as an effective irrigant in endodontic treatment. However, Assis et al. [36] found that CHX increases the free surface energy of dentin and decreases the contact angle of root canal sealers. However, because the surfactant and the CHX in QMix have an antagonistic effect on dentin surface energy and wettability, this antagonistic effect of surfactant and CHX may have made no changes in the chelation effect of the EDTA. This would explain why all canal sealers showed similar bonding strength to the root canal dentin when EDTA and QMix were used as the irrigants.
According to Deus et al. [40], when the smear layer is removed from the root canal wall, endodontic sealers penetrate to the dentinal tubules and increase adhesion to the root canal dentin. In the present study, the smear layer was not removed from the dentin wall in the distilled water group, as seen in SEM examination (Figure 1). All sealers showed lower bond strength values when irrigated with distilled water, because the sealers did not penetrate to the dentinal tubules. In the distilled water group, the Total Fill BC Sealer showed higher bond strength values than those of the other two sealers tested (p<0.05). Distilled water did not remove the smear layer, which has an unpredictable thickness and volume, since a great portion of it is water [41]. Total Fill BC Sealer absorbs water from the smear layer before the setting reaction occurs, and it can bond chemically with the smear layer. This would explain the higher bond strength values of Total Fill BC Sealer.
When the irrigants used were QMix, EDTA, and chitosan, the AH Plus and Total Fill BC sealers showed similar bond strength values, and both showed higher values than those of the MTA Fillapex sealer. As reported by Neto et al. [42], the fact that AH Plus was chemically bonds with the dentin molecules may be the result of the covalent bonding of epoxy links to the organic part of the dentin (the collagen amine groups released in the dentin). Previous studies have emphasized that the high bond strength of AH Plus could be due to the low polymerization stress of the sealer and its long-term dimensional stability [35,43]. This low polymerization stress and chemical bonding to the dentin may provide AH Plus higher bond strength than MTA Fillapex. In contrast, in MTA Fillapex, the Ca and OH ions released during the setting process due to the MTA content create an appetite formation [44] that may reduce the bond strength of the MTA Fillapex. This would explain why adhesive failure was the most frequent type of failure with MTA Fillapex.
The main component of dentin is hydroxyapatite, which has a hydroxy group. The setting reaction of the Total Fill BC Sealer, which is bioceramic-based, begins by absorbing water from the dentinal tubules. Calcium silicate hydrogel and hydroxyapatite compound are created after this reaction. The calcium silicate hydrogel binds chemically to the hydroxyapatite via the hydroxyl groups. The hydroxyapatite in the sealer follows a continuous process of crystal growth, and both compounds of the sealer form a strong chemical bond with the dentin. In addition, these sealers are capable of flowing into dentinal tubules without any shrinkage during the setting reaction of bioceramic-based sealers [21,45,46]. In this study, Total Fill BC Sealer showed bond strength values similar to those of AH Plus. This may be explained by the strong connection to the dentin of the various chemical mechanisms in both these sealers.
Conclusions
Chitosan may serve as an alternative chelating agent for use with various root canal sealers. It has both chelating effects and positive effects on the bonding of root canal sealers. Of the three sealers tested, Total Fill BC Sealer showed the highest bond strength value in the presence of smear layer. In addition, its bond strength and that of AH Plus were similar regardless of whether the smear layer was removed by EDTA, QMix, or chitosan. MTA Fillapex sealer had the lowest bond strength value in the neither smear presence nor absence. Further studies are needed to provide a better understanding of the effect of chitosan and its various abilities.
Conflicts of Interest
The authors have no conflicts of interest.
References
- Bystrom A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res 1981; 89: 321-328.
- Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dent Clin North Am 2010; 54: 291-312.
- McComb D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod 1975; 1: 238-242.
- Kara TA, Tuncer S, Siso SH. Effect of QMix irrigant on the microhardness of root canal dentine. Aust Dent J 2015; 60: 163-168.
- Dai L, Khechen K, Khan S, Gillen B, Loushine BA. The effect of QMix, an experimental antibacterial root canal irrigant, on removal of canal wall smear layer and debris. J Endod 2011; 37: 80-84.
- Qian W, Shen Y, Haapasalo M. Quantitative analysis of the effect of irrigant solution sequences on dentin erosion. J Endod 2011; 37: 1437-1441.
- Ordinola ZR, Bramante CM, Cavenago B. Antimicrobial effect of endodontic solutions used as final irrigants on a dentine biofilm model. Int Endod J 2012; 45: 162-168.
- Niu W, Yoshioka T, Kobayashi C. A scanning electron microscopic study of dentinal erosion by final irrigation with EDTA and NaOCl solutions. Int Endod J 2002; 35: 934-939.
- Rasimick BJ, Nekich M, Hladek MM, Musikant BL, Deutsch AS. Interaction between chlorhexidine digluconate and EDTA. J Endod 2008; 34: 1521-1523.
- Eliot C, Hatton JF, Stewart GP, Hildebolt CF, Jane GM. The effect of the irrigant QMix on removal of canal wall smear layer: an ex vivo study. Odontology 2014; 102: 232-240.
- Pai S, Thomas MS. The effect of QMix, an experimental antibacterial root canal irrigant, on removal of canal wall smear layer and debris. J Endod 2011; 37: 741-743.
- Stojicic S, Shen Y, Qian W, Johnson B, Haapasalo M. Antibacterial and smear layer removal ability of a novel irrigant, QMiX. Int Endod J 2012; 45: 363-371.
- Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB. A new solution for the removal of the smear layer. J Endod 2003; 29: 170-175.
- Kurita K. Chemistry and application of chitin and chitosan. Polym Degrad Stabil 1998; 59: 117-120.
- Akncbay H, Senel S, Ay ZY. Application of chitosan gel in the treatment of chronic periodontitis. J Biomed Mater Res B Appl Biomater 2007; 80: 290-296.
- Kishen A, Shi Z, Shrestha A, Neoh KG. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod 2008; 34: 1515-1520.
- Silva PV, Guedes DF, Pécora JD, da Cruz-Filho AM. Time-dependent effects of chitosan on dentin structures. Braz Dent J 2012; 23: 357-361.
- Barrieshi KM, Walton RE, Johnson WT. Coronal leakage of mixed anaerobic bacteria after obturation and post space preparation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997; 84: 310-314.
- Barbizam JV, Trope M, Tanomaru-Filho M, Teixeira EC, Teixeira FB. Bond strength of different endodontic sealers to dentin: push-out test. J Appl Oral Sci 2011; 19: 644-647.
- Borges AH, Orcati DMC, Dalla VR. Physicochemical properties and surfaces morphologies evaluation of MTA FillApex and AH plus. Sci World J 2014; 2014: 589732.
- Nagas E, Uyanik MO, Eymirli A, Cehreli ZC, Vallittu PK. Dentin moisture conditions affect the adhesion of root canal sealers. J Endod 2012; 38: 240-244.
- Del CPA, Bramante CM, Duarte MA. Chelating and antibacterial properties of chitosan nanoparticles on dentin. Restor Dent Endod 2015; 40: 195-201.
- Del CPA, Kishen A, Shrestha A. Antibacterial properties associated with chitosan nanoparticle treatment on root dentin and 2 types of endodontic sealers. J Endod 2015; 41: 1353-1358.
- Budiraharjo R, Neoh KG, Kang ET. Bioactivity of novel carboxymethyl chitosan scaffold incorporating MTA in a tooth model. Int Endod J 2010; 43: 930-939.
- Skidmore LJ, Berzins DW, Bahcall JK. An in vitro comparison of the intraradicular dentin bond strength of Resilon and gutta-percha. J Endod 2006; 32: 963-966.
- Orstavik D, Eriksen HM, Beyer-Olsen EM. Adhesive properties and leakage of root canal sealers in vitro. Int Endod J 1983; 16: 59-63.
- Torabinejad M, Higa RK, McKendry DJ, Pitt Ford TR. Dye leakage of four root end filling materials: effects of blood contamination. J Endod 1994; 20: 159-163.
- Formosa LM, Mallia B, Camilleri J. Push-out bond strength of MTA with antiwashout gel or resins. Int Endod J 2014; 47: 454-462.
- Hong ST, Bae KS, Baek SH. Effects of root canal irrigants on the push-out strength and hydration behavior of accelerated mineral trioxide aggregate in its early setting phase. J Endod 2010; 36: 1995-1999.
- Pane ES, Palamara JE, Messer HH. Critical evaluation of the push-out test for root canal filling materials. J Endod 2013; 39: 669-673.
- Rahimi S, Ghasemi N, Shahi S. Effect of blood contamination on the retention characteristics of two endodontic biomaterials in simulated furcation perforations. J Endod 2013; 39: 697-700.
- Saleh IM, Ruyter IE, Haapasalo M. The effects of dentine pretreatment on the adhesion of root-canal sealers. Int Endod J 2002; 35: 859-866.
- Baumgartner JC, Mader CL. A scanning electron microscopic evaluation of four root canal irrigation regimens. J Endod 1987; 13: 147-157.
- Hülsmann M, Rummelin C, Schafers F. Root canal cleanliness after preparation with different endodontic hand pieces and hand instruments: a comparative SEM investigation. J Endod 1997; 23: 301-306.
- Vilanova WV, Carvalho-Junior JR, Alfredo E. Effect of intracanal irrigants on the bond strength of epoxy resin-based and methacrylate resin-based sealers to root canal walls. Int Endod J 2012; 45: 42-48.
- Assis DF, Prado M, Simao RA. Evaluation of the interaction between endodontic sealers and dentin treated with different irrigant solutions. J Endod 2011; 37: 1550-1552.
- Ayranci LB, Koseoglu M. The evaluation of the effects of different irrigating solutions and laser systems on adhesion of resin-based root canal sealers. Photomed Laser Surg 2014; 32: 152-159.
- Tuncel B, Nagas E, Cehreli Z, Uyanik O, Vallittu P. Effect of endodontic chelating solutions on the bond strength of endodontic sealers. Braz Oral Res 2015; 29.
- Akcay I, Sen BH. The effect of surfactant addition to EDTA on microhardness of root dentin. J Endod 2012; 38: 704-707.
- De-Deus G, Accorsi-Mendonca T, de Carvalho e Silva L. Self-adjusting file cleaning-shaping-irrigation system improves root-filling bond strength. J Endod 2013; 39: 254-257.
- Cergneux M, Ciucchi B, Dietschi JM, Holz J. The influence of the smear layer on the sealing ability of canal obturation. Int Endod J 1987; 20: 228-232.
- Sousa NMD, Silva CFI, Marchesan MA. Ex vivo study of the adhesion of an epoxy-based sealer to human dentine submitted to irradiation with Er: YAG and Nd: YAG lasers. Int Endod J 2005; 38: 866-870.
- Lee KW, Williams MC, Camps JJ, Pashley DH. Adhesion of endodontic sealers to dentin and gutta-percha. J Endod 2002; 28: 684-688.
- Sarkar NK, Caicedo R, Ritwik P. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod 2005; 31: 97-100.
- Ersahan S, Aydin C. Dislocation resistance of iRoot SP, a calcium silicate-based sealer, from radicular dentine. J Endod 2010; 36: 2000-2002.
- Koch KA, Brave DG. Bioceramics, Part 2: The clinician’s viewpoint. Dent Today 2012; 31: 118, 120, 122-125.