Research Article - Biomedical Research (2017) Volume 28, Issue 15
Effect of astaxanthin on retinal pigment epithelial cells in high glucose: an in-vitro study
Desheng Fu1* and Xiaofeng Tian2
1Department of Ophthalmology, the Affiliated Union Hospital of Fujian Medical University, Fujian, PR China
2Department of Ophthalmology, Medical University of Tianjin, Tianjin, PR China
- *Corresponding Author:
- Desheng Fu
Department of Ophthalmology
The Affiliated Union Hospital of Fujian Medical University, PR China
Accepted date: July 11, 2017
Abstract
Background: Diabetic Retinopathy (DR) is now reported to be a leading cause of blindness in working age adults. The pathogenesis of diabetic retinopathy remains to be elucidated. Previous studies have reported that the Retinal Pigment Epithelium (RPE) cells were important for maintaining the visual system. The activated RPE cells can initiate proliferation and migration and secrete extracellular matrix molecules in diseases such as PDR. Besides, oxidative stress is reported to be one of the key progresses of the development of DR. Astaxanthin (ATX) is a carotenoid that is abundant in fishery products and is reported to have various beneficial bioactive properties for human and animal health. This study would provide the possible application of ATX on the treatment of DC.
Material and Method: In the present study, we evaluated the capacity of ATX (Sigma Chemical Company, St. Louis, MO, USA) in preventing high glucose induced abnormal activation and oxidative stress in human PRE cell. The in vitro culture with high glucose for 72 h was used to mimic the diabetic condition. ROS level was detected by ROS Fluorescent Probe-DHE. The control group was normal culture of RPE in high-glucose status.
Results: This is the first time to our knowledge that ATX in 50 μM pre-treatment can protect RPE cells from abnormal activation and oxidative stress induced by high glucose. The use of ATX could decrease the concentration of VEGF. It could be inferred that the protective effect might be conducted via the VEGF and its relative pathways.
Conclusions: We demonstrated that ATX could reduce the abnormal proliferation and oxidative stress induced by high glucose. The results extended our understandings on the molecular mechanisms of ATX inhibiting proliferation and oxidative stress in DR. ATX may be a potent inhibitor of VEGF and may be developed as a chemotherapeutic agent for DR therapeutics in the future.
Keywords
Retinal, Astaxanthin, Diabetic, VEGF
Introduction
Diabetes is one of the most important causes of retinal abnormalities that result in damage to the vasculature and neurons, and in severe cases, loss of vision itself. Diabetic Retinopathy (DR) is now reported to be a leading cause of blindness in working age adults [1]. DR is a chronic, progressive, sight-threatening disease associated with prolonged hyperglycemia. Improved glycemic control has been shown to inhibit the diabetes-induced loss of retinal capillaries in animals and the progression of DR [2].
The pathogenesis of diabetic retinopathy remains to be elucidated [3]. Previous studies have reported that the Retinal Pigment Epithelium (RPE) cells lie between Bruch’s membrane and the retina. They normally form a monolayer of cells, which create the outer blood-retinal barrier. RPE cells are important for maintaining the visual system. Normal RPE cells are quiescent without proliferation or migration. Breakdown of the outer blood-retinal barrier can expose RPE cells to various growth factors, neurotransmitter compounds, and cytokines in the subretinal space and in the vitreous, which can trigger the activation of RPE cells [4]. The activated RPE cells can initiate proliferation and migration and secrete extracellular matrix molecules in diseases such as PDR. Besides, oxidative stress is reported to be one of the key progresses of the development of DR [5]. The up-regulated oxidative agents, such as H2O2 and ROS, might play a key role in the progression of DR. The relative therapeutic measures that focused on the depression of the oxidative agents might be the potential applicable treatments [6].
Efforts to inhibit diabetic retinopathy and other complications of diabetes have focused on highly specific therapeutic approaches. For example, studies in animals have targeted specific pharmacologic targets or used genetic approaches to alter expression of single enzymes, such as iNOS (inducible isoform of nitric oxide synthase) [7] or superoxide dismutases [8]. These approaches have successfully inhibited the early stages of diabetic retinopathy in animals, but their translation to the clinic may be problematic. Astaxanthin (ATX) is a carotenoid that is abundant in fishery products and is reported to have various beneficial bioactive properties for human and animal health, including the prevention of cardiovascular disease, the promotion of immune responses, and antioxidative actions (Figure 1) [9,10]. These findings suggest a possible application of ATX in the management of DR.
In this study we tested the hypothesis that ATX might produce certain protective effect of DR. In this respect, we carried out in vitro experiments using in-vitro cellular model treated with high concentrations of glucose to mimic the diabetic condition. Advanced study was conducted to detect the effect of ATX on the cellular vitality of RPE cell and oxidative stress. This study would provide the possible application of ATX on the treatment of DC.
Materials and Methods
Chemicals
All chemicals used were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise specified. ATX (97% purity as determined by HPLC) was purchased from Sigma Chemical Company (St. Louis, MO, USA, No. SML0982). Rabbit polyclonal antibody against von mouse monoclonal antibodies against VEGF and β-actin were purchased from Santa Cruz (Santa Cruz, CA, USA).
Cell culture
Primary human RPE cells were isolated from donor eyes according to published procedures 18 and maintained in a 1:1 mixture of Medium 199 and Ham's F-12 medium, supplemented with 1 mM pyruvate, 200 U/ml penicillin, 100 μg/ml streptomycin, 10% heat inactivated fetal bovine serum (all reagents by PAA Laboratories, Coelbe, Germany), and 2 μg/ml insulin (Sigma-Aldrich, Munich, Germany). All subjects were treated in accordance with the Declaration of Helsinki.
Cell proliferation assay
RPE cell proliferation was measured with Methylthiazolyldiphenyl-Tetrazolium bromide (MTT) assay. For MTT assay, 50 μl of MTT solution (2 mg/ml; Sigma-Aldrich, Oakville, ON, Canada) was added to each well of RPE cells treated by a gradient time point and the wells were incubated at 37°C for 4 h. Mitochondrial and cytosolic dehydrogenases of living cells reduced the yellow tetrazolium salt (MTT) to a purple formazan dye that was then detected with spectrophotometry. After incubation, the MTT solution was aspirated, and 150 μl of DMSO was added for a period of 20 min. Optical densities of the supernatant were read at 550 nm using a microplate spectrophotometer (Bio-Tek Instruments, Inc., Winooski, VT). Absorbance was normalized to the untreated positive control cultures, which represented 100% viability.
Detection of ROS production
In general, the effect of ATX on ROS production in ARPE-19 cells was determined by a fluorometric assay using ROS Fluorescent DHE as the probe. This method is based on the oxidation from non-fluorescent DCFHDA to fluorescent 2’, 7’- Dichlorofluorescin (DCF). ARPE-19 cells (104 cells per well) in 48-well plates pre-treated with ATX were used for the ROS detection. The cells were washed with HBSS, then HBSS containing 10 μM DCFH-DA was added and incubation continued at 37°C for 45 min. The fluorescence was observed directly using a Fluorescence Microplate Reader.
Western blot assay
Cells were harvested and lysed with ice-cold lysis buffer (50 mM Tris-HCl, pH 6.8, 100 mM 2-ME, 2% w/v SDS, 10% glycerol). After centrifugation at 20000 Xg for 10 min at 4°C, proteins in the supernatants were quantified with BCA methods. After that the proteins were separated by 10% SDS PAGE, transferred to NC membrane (Amersham Bioscience, Buckinghamshire, U.K.). The membrane was then washed, and primary antibodies were detected with a secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature. After blocking with 10% nonfat milk in PBS, membranes were immunoblotted with antibodies as indicated, followed by HRP-linked secondary antibodies (Cell Signaling). The signals were detected by SuperSignal West Pico Chemiluminescent Substrate kit (Pierce, Rockford, IL) according to manufacturer’s instructions. Monoclonal rabbit anti-VEGF antibodies were purchased from Cell signaling, USA (No. 2445S). Protein levels were normalized to β-actin, using a rabbit anti-actin antibody (Santa Cruz, USA, No. sc-130657).
Statistical analysis
We used unpaired two-tailed Student’s t-test to compare differences in cell numbers, cloning efficiency, tumor weights, and other related parameters. We employed Chi-square test to compare incidence and latency. We used ANOVA (F-test) to compare differences in multiple groups. In all these analyses, a P<0.05 was considered statistically significant. All the analyses were conducted by SPSS 13.0 (IBM, Chicago, IL).
Results
Cell proliferation in high glucose condition
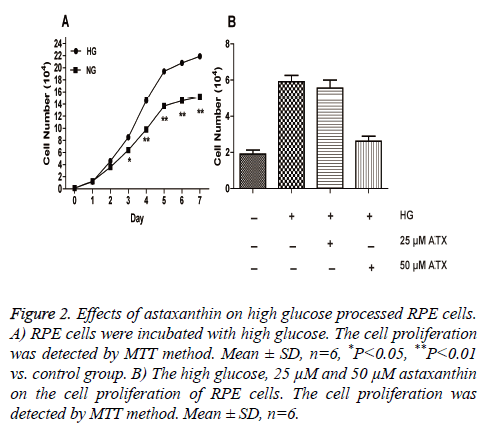
To evaluate the proliferation of primary cultured RPE cells after exposure to high glucose (30 mmol/L). When the cells were explored to high glucose, there cell proliferation was not different in d 1 and 2 while the cell proliferation significantly improved after d 3. Besides, the differences between the two groups were more and more significant from d’s 3-7 (Figure 2A).
Figure 2: Effects of astaxanthin on high glucose processed RPE cells. A) RPE cells were incubated with high glucose. The cell proliferation was detected by MTT method. Mean ± SD, n=6, *P<0.05, **P<0.01 vs. control group. B) The high glucose, 25 μM and 50 μM astaxanthin on the cell proliferation of RPE cells. The cell proliferation was detected by MTT method. Mean ± SD, n=6.
The effect of ATX on the cell proliferation exposed to high glucose
To evaluate the effect of ATX on the cell proliferation exposed in high glucose condition, we then treat the cells with ATX in different concentrations. Compared with the groups in the normal glucose condition, it was found that the cell proliferation in the high glucose condition was significantly improved. When 25 μM ATX was added, the cell proliferation didn’t significantly changed; however, the cell proliferation was significantly decreased when 50 μM ATX was used (Figure 2B).
The effect of ATX on the ROS content exposed to high glucose
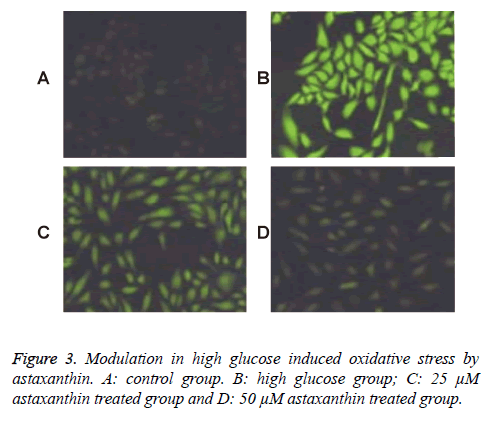
In the Figure 3, we could find that the ROS concentration of ROS was significantly increased in high glucose group (Figure 3). However, the ROS concentrations were significantly decreased in the groups that treated with 25 μM ATX and 50 μM ATX. Besides, compared with the groups with lower concentration, the groups with higher concentration would lead to a lower level of ROS in the RPE cells.
ATX down-regulates VEGF in high glucose at protein levels
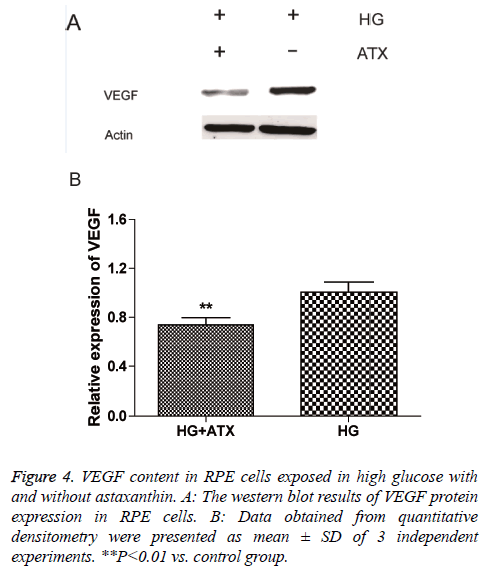
Protein levels of VEGF were detected by western-blot and it was found that the VEGF level were down-regulated after exposure to 50 μM of ATX (Figure 4). Compared with the control group (relative density=1), relative density of VEGF in the ATX treated group is 0.69 ± 0.08 (P<0.01).
Discussion
In the present study, we evaluated the capacity of ATX in preventing high glucose induced abnormal activation and oxidative stress in human PRE cell. This is the first time to our knowledge that ATX pre-treatment can protect RPE cells from abnormal activation and oxidative stress induced by high glucose. The use of ATX could decrease the concentration of VEGF. It could be inferred that the protective effect might be conducted via the VEGF and its relative pathways.
It has been reported that the proliferation and migration of RPEs cell were associated with DR, especially the PDR. In the progression of DR, the proliferation of RPE might be associated the breakdown of Blood-Retinal Barrier (BRB) [11]. In this study, the cell proliferation has been found to be increased in the high glucose condition. It could be inferred that high glucose induced abnormal activation of RPE cells might be one of the key influenced factors of the progressions of DR. Accordingly, the detection of the useful agents that prevent the abnormal activation of RPE cells in high glucose condition became the reasonable way for the detection of better therapeutic methods of DR.
Oxidative stress, which is induced by the increased accumulation of ROS and/or decreased antioxidant capacity, has an important role in the pathogenesis of DR. ROS are produced continuously as natural byproducts of the normal metabolism of oxygen and have important roles in redox signaling [12]. Increases in the generation of ROS in the diabetic retina have been confirmed by several groups. The NADPH oxidase family of enzymes (Noxs) are one of the major sources of ROS in many cell types and tissues, including the retina [13]. Among the seven subtypes of NADPH oxidase, NADPH oxidase-2 (Nox2) and -4 (Nox4) are two important modulators of redox signaling that are inducible in multiple cell types, including retinal cells, at the level of transcriptional expression [14]. Increased activity and expression of Nox2 and Nox4 have been found in the retinas of types 1 and 2 diabetic animal models, and have been related to increased oxidative stress in the diabetic retina. In this study, we found that the ROS content was increased in the high glucose condition. ROS generated by high glucose was considered as a causal link between elevated glucose and the other metabolic abnormalities important in the development of diabetic complications.
ATX is a naturally occurring carotenoid with strong antioxidant properties both in vitro and in vivo [15]. It has been reported that ATX restored the enzymatic antioxidant profile in salivary gland of alloxan-induced diabetic rats [16], and protected retinal cells against oxidative stress in vitro and in mice in vivo [17]. It was reported that in Proximal Tubular Epithelial Cells (PTECs), astaxanthin had a protective efficacy against several deleterious effects caused by high glucose exposure and proposed that ATX should be explored further as a potential antidiabetic remedy for the treatment of diabetic nephropathy. Dietary carotenoids exhibit various biological activities, including anti-oxidative activity. In particular, ATX was well known as a powerful antioxidant. In a previous study, an animal study was conducted to investigate whether ATX would protect against light-induced retinal damage. The result showed that ATX at 100 mg/kg inhibited the retinal dysfunction in terms of ERG and ONL loss and reduced the expression of apoptotic and 8-OHdG-positive cells induced by light exposure. Furthermore, ATX could protect against increased PI-positive cells and intracellular reactive oxygen species activity in retinal cells in animal model [18]. In this in vitro study, we could find that ATX could protect the high glucose induced oxidative stress in the cultured RPE cells.
The role of VEGF in the progression of DR has been discussed for a long time. In previous studies, VEGF could be associated with the proliferation [19] and oxidative stress [20] of RPE cells in the high glucose condition. As reported, VEGF was one of the most important growth factors of the RPE cells and the inhibition of VEGF in DR would lead to the depression of the proliferation of RPE cells. Besides, considering that the level of VEGF was significantly associated with the oxidative stress of DR [21]. The decrease of VEGF after the ATX treated group showed that ATX would lead a protective effect on the progression of DR. Retinal VEGF mRNA and protein expression increased in Streptozotosin (STZ) induced diabetic Sprague-Dawley rats. Systemic markers of oxidative stress and inflammation were elevated in insulin resistant and diabetic rats. Some oxidative stress and inflammatory markers (TNF-α, IL-6, ICAM-1, and IL1-β) were upregulated in the retina of STZ diabetic rats after 4 months of disease. In contrast, activation of NF-κB in the retina was observed in high fat fed non-diabetic and STZ-induced diabetic rats [22,23]. It could be inferred that ATX would lead to the depression of the inflammatory condition in the DR.
To sum up, we demonstrated that ATX could reduce the abnormal proliferation and oxidative stress induced by high glucose. The results extended our understandings on the molecular mechanisms of ATX inhibiting proliferation and oxidative stress in DR. ATX may be a potent inhibitor of VEGF and may be developed as a chemotherapeutic agent for DR therapeutics in the future.
References
- Tang J, Du Y, Petrash JM, Sheibani N. Deletion of aldose reductase from mice inhibits diabetes-induced retinal capillary degeneration and superoxide generation. PLoS One 2013; 8: 62081.
- Liu J, Feener EP. Plasma kallikrein-kinin system and diabetic retinopathy. Biol Chem 2013; 394: 319-328
- Yoshida Y, Yamagishi S, Matsui T. Protective role of pigment epithelium-derived factor (PEDF) in early phase of experimental diabetic retinopathy. Diabetes Metab Res Rev 2009; 25: 678-686.
- Klettner A, Westhues D, Lassen J. Regulation of constitutive vascular endothelial growth factor secretion in retinal pigment epithelium/choroid organ cultures: p38, nuclear factor kappaB, and the vascular endothelial growth factor receptor-2/phosphatidylinositol 3 kinase pathway. Mol Vis 2013; 19: 281-291.
- Longo-Mbenza B, Mvitu Muaka M, Masamba W. Retinopathy in non-diabetics, diabetic retinopathy and oxidative stress: a new phenotype in Central Africa? Int J Ophthalmol 2014; 7: 293-301.
- Kundu D, Mandal T, Nandi M. Oxidative stress in diabetic patients with retinopathy. Ann Afr Med 2014; 13: 41-46.
- Leal EC, Manivannan A, Hosoya K. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci 2007; 48: 5257-5265.
- Mohammedi K, Bellili-Munoz N, Driss F. Manganese superoxide dismutase (SOD2) polymorphisms, plasma advanced oxidation protein products (AOPP) concentration and risk of kidney complications in subjects with type 1 diabetes. PLoS One 2014; 9: 96916.
- Asker D, Awad TS, Beppu T, Ueda K. A novel radio-tolerant astaxanthin-producing bacterium reveals a new astaxanthin derivative: astaxanthin dirhamnoside. Methods Mol Biol 2012; 892: 61-97.
- Barros MP, Marin DP, Bolin AP. Combined astaxanthin and fish oil supplementation improves glutathione-based redox balance in rat plasma and neutrophils. Chem Biol Interact 2012; 197: 58-67.
- Hernandez C, Garcia-Ramirez M, Simo R. Overexpression of hemopexin in the diabetic eye: a new pathogenic candidate for diabetic macular edema. Diabetes Care 2013; 36: 2815-2821.
- Ahmado A, Carr AJ, Vugler AA. Induction of differentiation by pyruvate and DMEM in the human retinal pigment epithelium cell line ARPE-19. Invest Ophthalmol Vis Sci 2011; 52: 7148-7159.
- Yang J, Dong S, Jiang Q. Changes in expression of manganese superoxide dismutase, copper and zinc superoxide dismutase and catalase in Brachionus calyciflorus during the aging process. PLoS One 2013; 8: 57186.
- Jones KJ, Chetram MA, Bethea DA. Cysteine (C)-X-c receptor 4 regulates NADPH oxidase-2 during oxidative stress in prostate cancer cells. Cancer Microenviron 2013.
- Patel H, Chen J, Das KC, Kavdia M. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc Diabetol 2013; 12: 142.
- Ishiki M, Nishida Y, Ishibashi H. Impact of divergent effects of astaxanthin on insulin signaling in L6 cells. Endocrinology 2013; 154: 2600-2612.
- Naito Y, Uchiyama K, Mizushima K. Microarray profiling of gene expression patterns in glomerular cells of astaxanthin-treated diabetic mice: a nutrigenomic approach. Int J Mol Med 2006; 18: 685-695.
- Naito Y, Uchiyama K, Aoi W. Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic db/db mice. Biofactors 2004; 20: 49-59.
- Otsuka T, Shimazawa M, Nakanishi T. Protective effects of a dietary carotenoid, astaxanthin, against light-induced retinal damage. J Pharmacol Sci 2013; 123: 209-218.
- Liegl R, Koenig S, Siedlecki J. Temsirolimus inhibits proliferation and migration in retinal pigment epithelial and endothelial cells via mTOR inhibition and decreases VEGF and PDGF expression. PLoS One 2014; 9: 88203.
- Izuta H, Matsunaga N, Shimazawa M. Proliferative diabetic retinopathy and relations among antioxidant activity, oxidative stress, and VEGF in the vitreous body. Mol Vis 2010; 16: 130-136.
- Zhu Y, Zhang XL, Zhu BF, Ding YN. Effect of antioxidant N-acetylcysteine on diabetic retinopathy and expression of VEGF and ICAM-1 from retinal blood vessels of diabetic rats. Mol Biol Rep 2012; 39: 3727-3735.
- Mima A, Qi W, Hiraoka-Yamomoto J. Retinal not systemic oxidative and inflammatory stress correlated with VEGF expression in rodent models of insulin resistance and diabetes. Invest Ophthalmol Vis Sci 2012; 53: 8424-8432.