Research Article - Biomedical Research (2017) Volume 28, Issue 8
Efavirenz in combination with n-acetyl cysteine shows improved outcome in treatment of hepatitis B
Lili Liu1, Longyan Chen2, Fuhai Li1, Yan Zhang1 and Jie Yang1*1Children’s Medical Center, Qilu Hospital of Shandong University, Jinan, Shandong 250012, P.R. China
2Department of Hepatology, Qilu Hospital of Shandong University, Jinan, Shandong 250012, P.R. China
- *Corresponding Author:
- Jie Yang
Children’s Medical Center
Qilu Hospital of Shandong University
Jinan, Shandong 250012, P.R. China
Accepted on August 06, 2016
Abstract
Hepatitis B virus (HBV) infection is one of the leading causes of liver disorders such as liver cirrhosis and hepatocellular carcinoma. The prognosis of hepatitis B in patients with HIV co-infection is poor and chances of spontaneous clearance of HBV infection is less likely. To investigate if n-acetyl cysteine (NAC) would protect liver from toxic effects of efavirenz (EFVZ) as a possible combination therapy for hepatitis B. In vitro studies were performed using the HepG2 2.2.15 cell lines and duck model of hepatitis B (Guangxi Small Partridge; n=48), animals were randomly divided into eight groups (n=6 in each group). The drug groups were treated with three dosages of efavirenz (25, 50, 100 mg/kg/day) and three dosages of three dosages of efavirenz (25, 50, 100 mg/kg/day) plus N-acetyl cysteine (NAC) (20 mM). Both efavirenz and NAC inhibited the growth of HepG2.2.15 cells at high concentrations in a dosedependent manner. The treatment of HepG2.2.15 cells transfected with HBV, with efavirenz for 24 h also exhibited a dose dependent inhibitory effect on the secretion of HBsAg and HBeAg antigens. In duck HBV infection model, efavirenz plus NAC not only significantly reduced serum HBV DNA levels but also improved ALT and AST profile (p=0.03). Our study provides useful evidence that co-administration of NAC with efavirenz protects the toxic effect of efavirenz, in patients with hepatitis B infection.
Keywords
HIV, Hepatitis B, Antioxidant.
Introduction
Hepatitis B virus (HBV) infection is one of the most commonly observed viral infection and leading cause of acute and chronic liver disorders such as liver cirrhosis and hepatocellular carcinoma. Out of 400 million people infected with HBV worldwide, around 6.9 million infected individuals and 120 million asymptomatic carriers have been reported in China [1,2]. Studies also estimate that out of 350-400 million of world’s population of HBV patients, 75% of the population lives in Asia. Each year around 1 million liver-related deaths are attributed to HBV infection related liver disorders [3].
The prognosis of hepatitis B in patients with HIV co-infection is poor and chances of spontaneous clearance of HBV infection is less likely [4]. In such patients the progression of acute hepatitis B into chronic infection is four times more likely, particularly in patients with low CD4 count [5]. In patients with chronic hepatitis B and HIV co-infection the incidence of progression into cirrhosis, hepatocellular carcinoma and end-stage liver disease is much higher [5]. Increase in liver transaminases often leads to discontinuation of the treatment in 6% to 30% of the patients with HIV and hepatitis B co-infection, which further leads to development of treatment resistance [6].
Efavirenz (EFVZ) is a first-generation benzoxazinone, nonnucleoside reverse transcriptase inhibitor (NNRTI), widely used for the treatment of HIV infection and also post exposure prophylaxis [7,8]. Efavirenz is extensively metabolized, with cytochrome P450 (CYP), CYP2B6-catalyzed primary (to 8- hydroxyefavirenz) therefore associated with number of drug-drug interactions [7,8]. In patients with HBV and HIV infection treatment with efavirenz is associated with liver toxicity, which leads to its limited use. Use of antioxidant can be an effective strategy to counter the cytotoxicity of therapeutic agents. N-acetyl cysteine (NAC) is a popular antioxidant widely known to be used in combination with cytotoxic drugs.
NAC is a metabolite of amino acid cysteine and a potent antioxidant. NAC is used in wide range of illness and also used as dietary supplement. Intravenous NAC is approved for use for the treatment of acetaminophen overdose and acrylonitrile and methacrylonitrile poisonings [9]. In the present study, we aimed to investigate the effect of n-acetyl cysteine and efavirenz combination as a possible treatment of HIV and hepatitis B co-infection. We also evaluated if NAC would decrease the toxicity of efavirenz on the normal liver cells.
Materials and Methods
Chemicals
Efavirenz (EFVZ) (99.0% purity) was purchased from Xing Cheng Chemphar (China). Milli-Q water from a Millipore Water Purification System was used where distilled water was required. Efavirenz was dissolved in DMSO (≤ 1%) and then diluted with culture medium for in vitro experiments. For animal experiments efavirenz was dissolved in distilled water and later diluted with normal saline for the animal tests. Lamivudine (LMVD) was obtained from GlaxoSmithKline (Fujian, P.R. China) and served as the positive control.
Cell culture
HepG2.2.15 (clonal cells derived fromhuman hepatoma cell line G2) cells were obtained from the Chinese Academy of Medical Sciences (China) and maintained in modified eagle medium (MEM) supplemented with 10% fetal bovine serum (FBS) and 380 μg/ml of G418, 50 U/ml of kanamycin, and 0.03% L-glutamine (Mercator, China) at 37°C in a 5% CO2 atmosphere with 100% humidity.
Cell toxicity studies
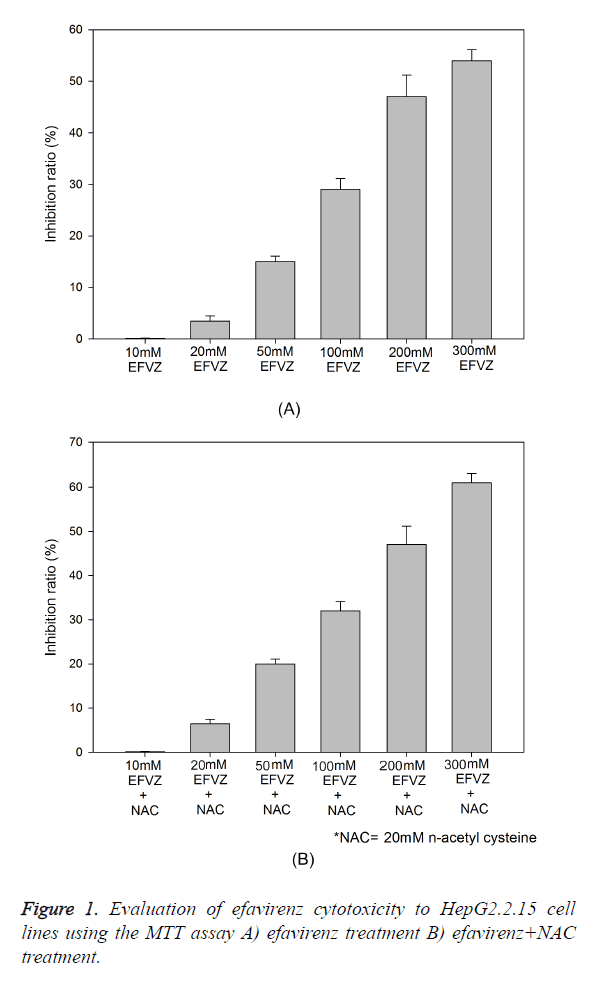
The cytotoxic effect of Efavirenz towards HepG2 2.2.15 was evaluated using the MTT assay (10). Logarithmically growing cells were seeded in 96-well culture plates at a density of 1 × 105 cells/ml (200 μl/well). They were cultured for 24 h and then treated with various concentrations of efavirenz (20, 42.1, 63.5, 128.2, 182.4 μM). OD values were read at 450 nm after 24 h and the percent of cell death was calculated.
Treatment of HepG2.2.15 cells with efavirenz
HepG2.2.15 cells were seeded at a density of 1 105 cells/ml (200 μl/well) in 96-well plates and maintained at 37°C for 24 h, followed by treatment with various concentrations (20, 42.1, 63.5, 128.2, 182.4 μM) of efavirenz or 40 μM lamivudine (3TC). After 24 h of treatment, the supernatants from each group were collected independently to determine the secretion of albumin and the levels of HBV surface antigen (HBsAg), HBV e antigen (HBeAg) and HBV DNA.
Determination of HBsAg and HBeAg
HBsAg and HBeAg were simultaneously detected using ELISA kits (Rongsheng Biotechnology Co. Ltd., Shanghai, China) according to the manufacturer's instructions. The plates were read directly with a microplate reader at a wavelength of 450 nm. The inhibition ratio (%) was calculated as follows: (OD control-OD sample)/OD control *100%.
Detection of HBV DNA by fluorescence quantitative- PCR assay
To further evaluate the inhibitory effects of efavirenz on HBV replication, the extracellular and intracellular HBV DNA levels were determined by fluorescence quantitative PCR (FQ-PCR).
The culture supernatants were used to measure extracellular HBV DNA and the cells were harvested for the analysis of intracellular HBV DNA. The viral DNA was extracted using Viral DNA Extraction Kit (TaKaRa, Dalian, China). Briefly, HBV DNA was extracted and amplified with a Step One Plus Real-Time PCR system using FastStart Universal SYBR Green Master (ROX). The forward primer was 5’-AAC CAT TGA AGC AAT CAC TAG AC-3’, and the reverse primer was 5’- ATC TAT GGT GGC TGC TCG AAC TA-3’ [10,11]. The PCR was carried out in a 25 μl reaction volume containing 12.5 μl 2 SYBR Green Master (ROX), 1.0 μl 10 μM forward primer, 1.0 μl 10 μM reverse primer 8.5 μl ddH2O and 2.0 μl HBV DNA. The thermal program comprised of an initial denaturation at 95°C for 10 min followed by 40 amplification cycles with each of the two following steps: 95°C for 15 s and 60°C for 1 min. DHBV DNA was quantified using a standard curve. In this assay, the linear range was 1 × 105-1 × 1011 copies/ml. The inhibition ratio was calculated through the following formula: (negative control-treated test sample)/negative control × 100%.
Animal studies
All animal procedures were performed as per the guidelines of the Care and Use of Laboratory Animals and were approved by the Animal ethical Care and Use Committee of the Qilu Hospital of Shandong University, China. All experimental procedures were conducted in conformity with institutional guidelines and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals. Both male and female ducks (Guangxi Small Partridge) were used (n=48). Each one day old duck was injected into its tibial vein with 0.2 ml of serum from ducks with positive DHBV DNA serology [1]. The drug treatment experiment was carried out 7 days after ducks were infected with DHBV, and then the positive ducks were randomly divided into eight groups (n=6 in each group). The drug groups were treated with three dosages of efavirenz (25, 50, 100 mg/kg/day) and three dosages of three dosages of efavirenz (25, 50, 100 mg/kg/day) plus N-acetyl cysteine (20 mM). The positive control group received 50 mg/kg/day LMVD and the control group received normal saline. Drug efavirenz and LMVD were administered orally once daily for 14 days continuously. Animals in the efavirenz+NAC treatment group received ad lib drinking water supplemented with 20 mM NAC and remained on supplemented water until sacrifice. Blood samples were taken at initiation of treatment (T0), the seventh day of treatment (T7), the fourteenth day of treatment (T14) and the third day (P3) of post-treatment follow-up. The serum was stored at 80°C until analysis. At the end of the experiment, the ducks were sacrificed, and the liver tissues were removed and fixed in a 10% formalin solution for histopathologic examination.
Detection of serum Duck Hepatitis B Virus (DHBV) DNA
DHBV DNA was detected at 0, 7, 14 days, and 3 days after cessation of treatment by FQ-PCR as described previously. The serum DNA was extracted using Viral DNA Extraction Kit (TaKaRa, Dalian, China). HBV DNA inhibition rate (%)=(copy number of the control-copy number of the study sample)/copy number of the control *100%.
Determination of serum ALT and AST
Serum samples were collected from each group at days 0, 7, 14 of the treatment and day 3 post-treatment. The serum levels of alanine transaminase (ALT) and aspartate aminotransferase (AST) were measured by AST and ALT detection kits (Shanghai Zhicheng Bioengineering Institute, Shanghai, P.R. China).
Histopathological examination of duck liver
The DHBV-positive ducks were treated with efavirenz and LMVD (i.g.) once daily for 14 days. On day 3 of post-treatment, the animals were euthanized and the liver tissues were removed. A portion of each harvested liver tissue was instantly fixed in 10% phosphate-buffered formalin. Then, this portion was processed by embedding in paraffin, sectioning into 5 μm pieces. The samples were stained with haematoxylin and eosin (H & E), and examined using light microscopy for histopathological examination.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software for windows. One-way analysis of variance (ANOVA) was used to compare the means among different groups, and the Tukey’s test was used to conduct multiple post-hoc comparisons. Results are expressed in means ± S.D. A P value <0.05 was considered statistically significant.
Results
Cytotoxic effect of efavirenz on HepG2 2.2.15 cell line
The cytotoxicity of efavirenz was determined using fresh culture medium containing serial 1:2 dilutions of efavirenz. The inhibition ratio of the HepG2.2.15 cells exposed to different concentrations of efavirenz is shown in Figure 1. There was an increase in the inhibition ratio percentage when NAC (20 mM) was added to the same concentration of EFVZ in HepG2.2.15 cells (P=0.061). The 50% cytotoxic concentration (TC50) was 295.3 μM, and the maximum nontoxic concentration (TC0) was 8.1 μM. These results were used to determine the dose range of efavirenz for the subsequent experiments. The maximum inhibition achieved by EFVZ was 58% and EFVZ plus NAC was 63.6% at dose of 300 mM of EFVZ (P=0.046).
Antiviral effects of efavirenz in HepG2.2.15 cells
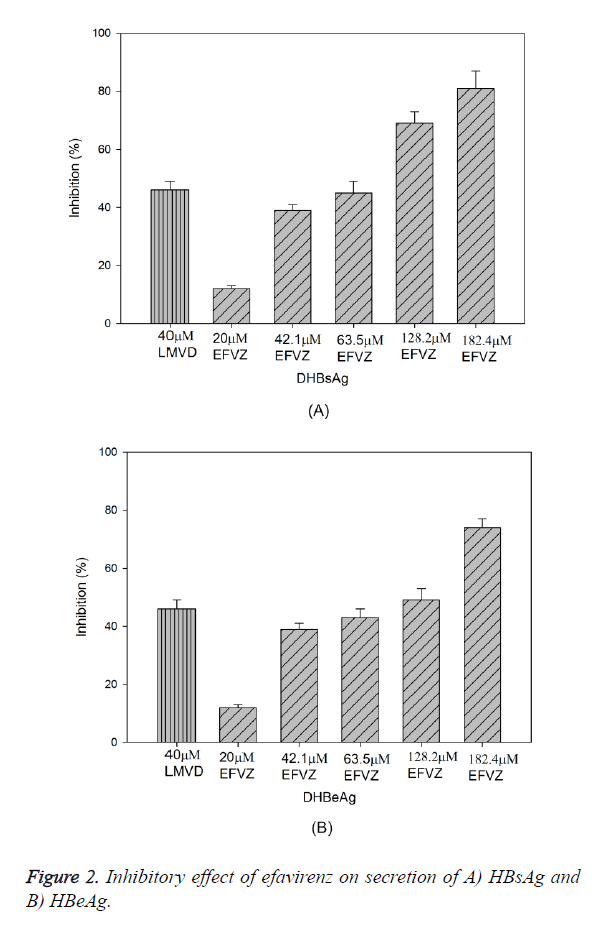
Treatment of HepG2.2.15 cells with efavirenz at various concentrations for 24 h resulted in significant reduction of HBsAg and HBeAg secretion in a dose-dependent manner, with IC50 values of 17.4 μM and 63.9 μM, respectively (Figure 2A). At the concentration of 42.1, 63.5, 128.2 and 182.4 μM, efavirenz showed significant inhibitory effects on HBsAg secretion, with the highest inhibition at 89.3%. Similarly to the HBeAg secretion, the secretion of HBeAg was significantly reduced with the highest inhibition at 79.5%. In the same experiment, lamivudine (40 μM) suppressed the secretion of HBsAg and HBeAg by 48.5% and 46.8%, respectively.
In vivo anti-HBV activity of efavirenz in duck model
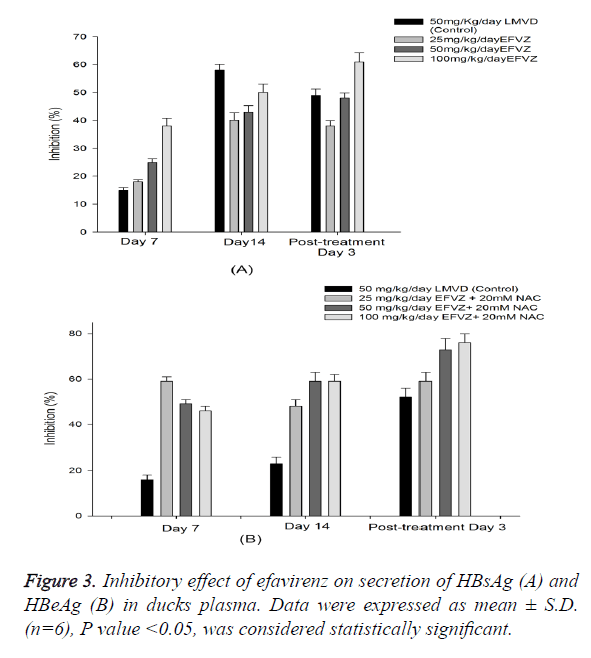
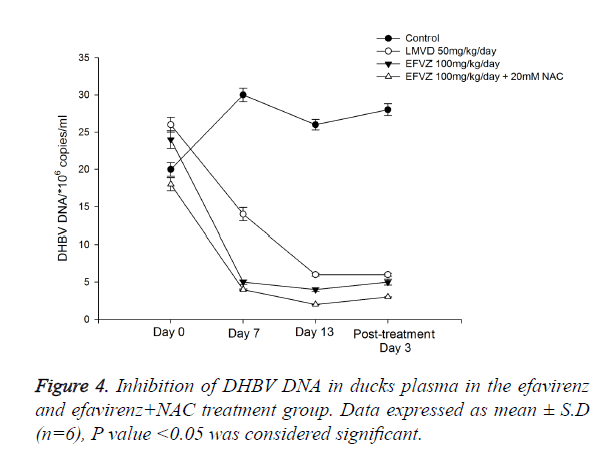
We fed the ducks with efavirenz at various concentrations once daily for 14 days, no toxicity was observed. To examine the in vivo anti-HBV activity of efavirenz, we first examined the HBsAg and HBeAg secretion in serum. To investigate the anti- HBV activity of efavirenz in ducks, the plasma DHBV DNA levels of the infected ducks with and without treatment were evaluated by FQ-PCR. During this experimental period, no significant side effects were observed in animals receiving antiviral therapy, while one third of ducks in the control group died. After treatment with efavirenz and LMVD for 7 and 14 days, the level of DHBV DNA of each group decreased significantly (P<0.05). As shown in Figure 4, the treatment with efavirenz at the dose of 25, 50 and 100 mg/kg/day for 14 days exhibited a time and dose dependent inhibitory effect on DHBV DNA level. The rebound of the DHBV DNA levels in efavirenz plus NAC treated ducks was to a less extent as compared with the LMVD treated group. No significant differences were observed in the DHBV DNA levels of any of the controls (Figure 3).
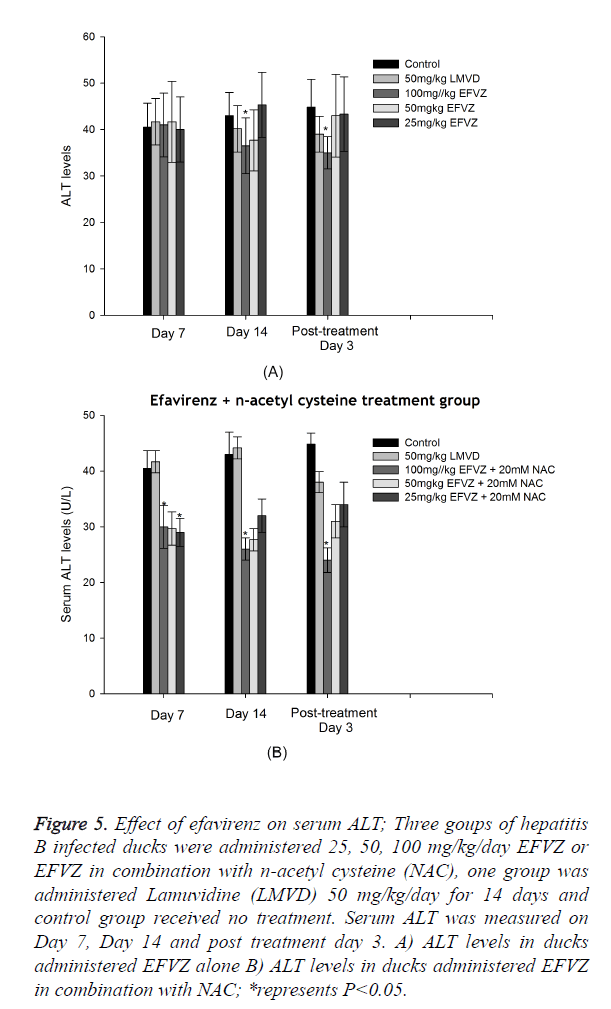
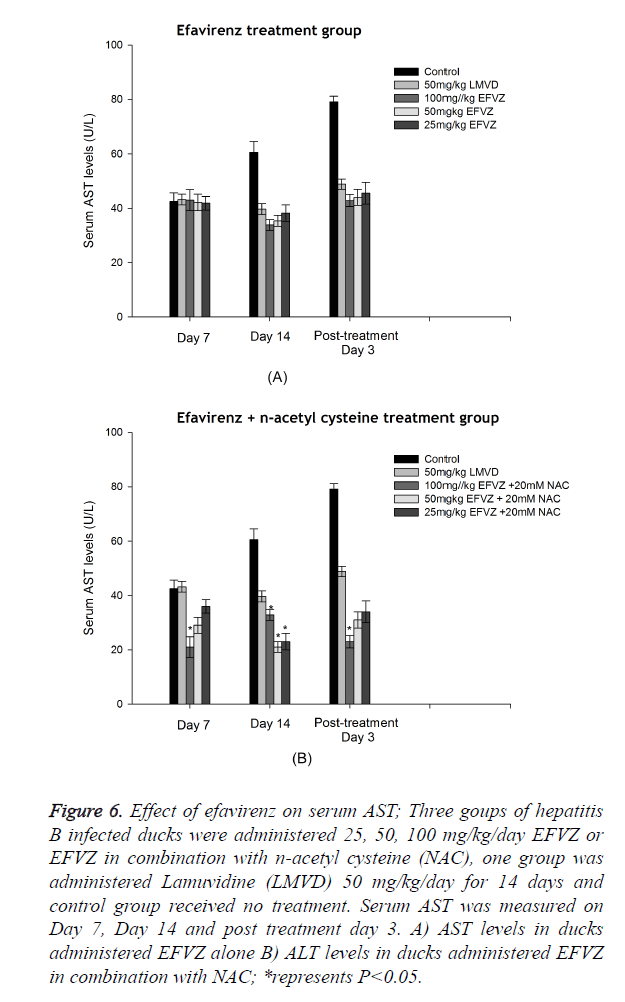
Analysis of ALT, AST levels in serum
DHBV infection resulted in a significant hepatic damage. The ALT, AST levels of model control group increased evidently compared to normal group. Oral administration of efavirenz at three different doses for 7 and 14 days resulted in lower levels of ALT and AST in serum (Figures 5 and 6).
Figure 5. Effect of efavirenz on serum ALT; Three goups of hepatitis B infected ducks were administered 25, 50, 100 mg/kg/day EFVZ or EFVZ in combination with n-acetyl cysteine (NAC), one group was administered Lamuvidine (LMVD) 50 mg/kg/day for 14 days and control group received no treatment. Serum ALT was measured on Day 7, Day 14 and post treatment day 3. A) ALT levels in ducks administered EFVZ alone B) ALT levels in ducks administered EFVZ in combination with NAC; *represents P<0.05.
Figure 6. Effect of efavirenz on serum AST; Three goups of hepatitis B infected ducks were administered 25, 50, 100 mg/kg/day EFVZ or EFVZ in combination with n-acetyl cysteine (NAC), one group was administered Lamuvidine (LMVD) 50 mg/kg/day for 14 days and control group received no treatment. Serum AST was measured on Day 7, Day 14 and post treatment day 3. A) AST levels in ducks administered EFVZ alone B) ALT levels in ducks administered EFVZ in combination with NAC; *represents P<0.05.
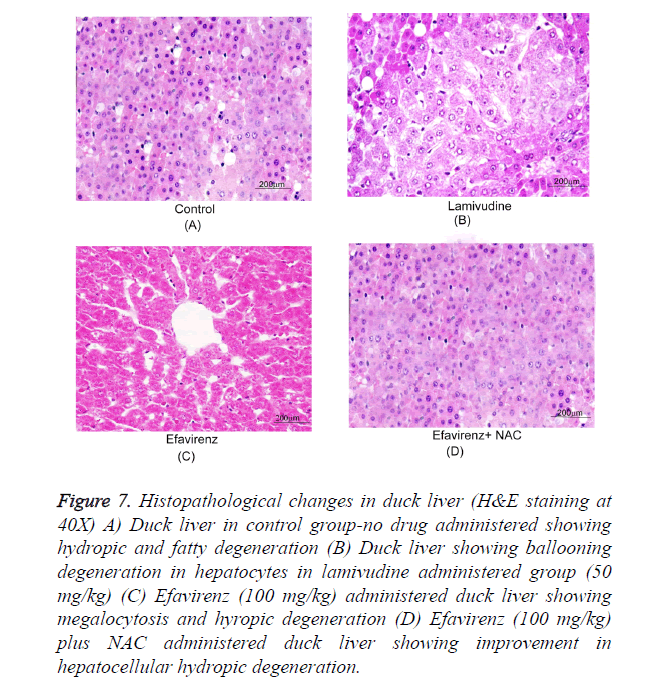
Histopathological examination of the duck livers
Typical photographs of liver sections by light microscopy show normal hepatocyte structure, and single layer of cells arranged around the central vein in a radical pattern. The following typical pathological characteristics were obvious in the model group: revealing massive ballooning degeneration, hepatocyte, loosening necrosis and inflammatory cell infiltration. Samples treated with efavirenz, on the other hand, exhibited dose dependent improvement of the hepatocellular architecture (Figure 7). It is worth noting that efavirenz at 50 and 100 mg/kg/day resulted in a more significant improvement than 3TC at 50 mg/kg/day. The results of the evaluation of all of the samples are summarized in Table 1.
Figure 7. Histopathological changes in duck liver (H&E staining at 40X) A) Duck liver in control group-no drug administered showing hydropic and fatty degeneration (B) Duck liver showing ballooning degeneration in hepatocytes in lamivudine administered group (50 mg/kg) (C) Efavirenz (100 mg/kg) administered duck liver showing megalocytosis and hyropic degeneration (D) Efavirenz (100 mg/kg) plus NAC administered duck liver showing improvement in hepatocellular hydropic degeneration.
| Group | No. of animals (n) | Pathological Scope | |||
|---|---|---|---|---|---|
| No lesion | Light (<1/3) | Medium (1/3, 2/3) | Serious (>2/3) | ||
| Control | 6 | 0 | 0 | 2 | 4 |
| Lamivudine group | 6 | 1 | 0 | 3 | 2 |
| Efavirenz group | 6 | 0 | 0 | 1 | 5 |
| Efavirenz and n-acetyl cysteine group | 6 | 3 | 2 | 1 | 0 |
Table 1. Summary of histopathological changes in duck livers.
The liver sections were evaluated primarily on the basis of their hepatocytic degeneration, chronic inflammation and cell infiltration. Efavirenz treatment samples exhibited dose-dependent improvement of the hepatocellular architecture. Although treatment with 25 mg/kg/day efavirenz did not have a significant effect compared with 3TC treatment, efavirenz at 50 and 100 mg/kg/day resulted in a more significant improvement than 3TC at 50 mg/kg/day.
Discussion
The anti-HIV drugs such as nevirapine and efavirenz are known to cause hepatocellular damage [12-14]. Therefore, in spite of showing efficacy in clearing hepatitis B virus (HBV) there drugs have limited usefulness in clinical setting in HIV patients with HBV co-infection. In addition, patients with HBV already have varied extend of liver damage and possess treat of relapse after cessation of therapy [15]. Compared with traditional nucleoside drugs, efavirenz is a non-nucleoside reverse transcriptase inhibitor (NNRTI) and inducer of the cytochrome P450 system therefore metabolised by liver. Efavirenz is used as part of highly active antiretroviral therapy (HAART) regimen, in combination with other retroviral drugs. In patients with deranged liver function this often causes serious adverse events [16].
In the present study, the hepatoprotective effect of N-acetyl cysteine (NAC) was utilised to evaluate the beneficial effect on lowering efavirenz induced hepatotoxicity, in vitro and in vivo for the first time. Using HepG2 2.2.15 cells, we showed that cytotoxicity was observed after the administration of low concentrations of efavirenz with TC0 of 9.7 μM. Both efavirenz and NAC inhibited the growth of HepG2.2.15 cells at high concentrations in a dose-dependent manner. The treatment of HepG2.2.15 cells transfected with HBV, with efavirenz for 24 h also exhibited a dose dependent inhibitory effect on the secretion of HBsAg and HBeAg antigens. Treatment with efavirenz, as well as efavirenz with NAC for 24 h, showed lower levels of extracellular and intracellular HBV DNA than negative control. Compared with LMVD (40 μM), efavirenz and concomitantly administration of NAC showed stronger inhibitory activity at the concentration of 128.2 or 182.4 μM.
To demonstrate the in vivo anti-HBV activity of efavirenz, and investigate if concomitant administration of NAC has hepatoprotective effect, we investigated the duck model of hepatitis B, a widely used system for the study of in vivo activity of anti-hepatitis agents [17,18]. In duck HBV infection model, efavirenz plus NAC not only significantly reduced serum HBV DNA levels but also improved ALT and AST profile. AST and ALT levels are associated with extent of the liver damage, are are commonly considered as good indicators of hepatocyte integrity [19]. Compared with LMVD (40 mg/kg/day) efavirenz and NAC combination showed inhibition of the rebound of DHBV DNA more effectively. Oral administration of efavirenz (25, 50, 100 mg/kg/day) in combination with NAC 20 mM (25, 50, 100 mg/kg/day) significantly reduced the elevated ALT and AST levels in serum. It is also important to note that efavirenz (50, 100 mg/kg/day) plus NAC group showed marked improvement in histopathological score.
Conclusion
Our study provides useful evidence that co-administration of NAC with efavirenz protects the liver from the toxic effect of efavirenz mono or combination therapy, in patients with hepatitis B infection. This can further be useful in treatment of patients with HIV and HBV co-infection. One limitation of this study is the absence of NAC alone group, which could have helped in identifying the contribution of NAC towards therapeutic efficacy. Further studies and validation in clinical settings are required to include NAC in the treatment protocol for HIV positive patients with hepatitis B infection.
Acknowledgement
This study was supported by the Foundation for Outstanding Young Scientist in Shandong Province (BS2011YY018)
References
- Li H, Tian D, Wu H, Chen M, Chen A. The effect of Gankang Suppository on duck hepatitis B virus, serum biochemistry and liver histology in ducklings. J Huazhong Univ Sci Technolog Med Sci 2008; 28: 421-425.
- Luo Z, Xie Y, Deng M, Zhou X, Ruan B. Prevalence of hepatitis B in the southeast of China: a population-based study with a large sample size. Eur J Gastroenterol Hepatol 2011; 23: 695-700.
- McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis 2005; 25: 3-8.
- Gatanaga H, Yasuoka A, Kikuchi Y, Tachikawa N, Oka S. Influence of prior HIV-1 infection on the development of chronic hepatitis B infection. Eur J Clin Microbiol Infect Dis 2000; 19: 237-239.
- Mallet V, Vallet-Pichard A, Pol S. The impact of human immunodeficiency virus on viral hepatitis. Liver Int 2011; 31: 135-139.
- Chauvel O, Lacombe K, Bonnard P, Lascoux-Combe C, Molina JM, Miailhes P. Risk factors for acute liver enzyme abnormalities in HIV-hepatitis B virus-coinfected patients on antiretroviral therapy. Antivir Ther 2007; 12: 1115-1126.
- Maggiolo F. Efavirenz: a decade of clinical experience in the treatment of HIV. J Antimicrob Chemother 2009; 64: 910-928.
- Rakhmanina NY, van den Anker JN. Efavirenz in the therapy of HIV infection. Expert Opin Drug Metab Toxicol 2010; 6: 95-103.
- Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opin Biol Ther 2008; 8: 1955-1962.
- Ferrari M, Fornasiero MC, Isetta AM. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods 1990; 131: 165-172.
- Duflot A, Mehrotra R, Yu SZ, Barraud L, Trepo C, Cova L. Spectrum of liver disease and duck hepatitis B virus infection in a large series of Chinese ducks with hepatocellular carcinoma. Hepatology 1995; 21: 1483-1491.
- Torresi J, Locarnini S. Antiviral chemotherapy for the treatment of hepatitis B virus infections. Gastroenterology 2000; 118: S83-103.
- Sims KA, Woodland AM. Entecavir: a new nucleoside analog for the treatment of chronic hepatitis B infection. Pharmacotherapy 2006; 26: 1745-1757.
- Zheng LY, Zang LM, Yang QH, Yu WQ, Fang XZ, Zhang YH. Anti-hepatitis B virus activity of alpha-DDB-DU, a novel nucleoside analogue, in vitro and in vivo. Eur J Pharmacol 2013; 702: 258-263.
- Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology 2002; 35: 182-189.
- van LF, Phanuphak P, Ruxrungtham K, Baraldi E, Miller S, Gazzard B, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet 2004; 363: 1253-1263.
- Guha C, Mohan S, Roy-Chowdhury N, Roy-Chowdhury J. Cell culture and animal models of viral hepatitis. Part I: hepatitis B. Lab Anim 2004; 33: 37-46.
- Wang CY, Giambrone JJ, Smith BF. Detection of duck hepatitis B virus DNA on filter paper by PCR and SYBR green dye-based quantitative PCR. J Clin Microbiol 2002; 40: 2584-2590.
- Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008; 245: 194-205.