Research Article - Journal of Food Microbiology (2018) Journal of Food Microbiology (Special Issue 1-2018)
Edible transparent coating of irradiated oligo-chitosan to preserve aesthetic view and taste of litchi fruit.
Sakila Jesmin1,2, Abdullah-Al-Jubayer1,4, Tonmoy Debnath5, Jahid M M Islam1,3,6, M Samsur Rahaman1,3, A.H. M. Kamal1, M Saifur Rahaman1, Shariff E Kabir2 and Mubarak A khan1,3*
1Institute of Radiation and Polymer Technology, Bangladesh Atomic Energy Commission, P.O. Box 3787, Dhaka-1000, Bangladesh
2Department of Chemistry, Jahangirnagar University, Savar, Dhaka-1342, Bangladesh
3Jute Polymer Unit, Bangladesh Jute Mills Corporation, Ministry of Textiles and Jute, Bangladesh
4Department of Biotechnology and Genetic Engineering, Bangabandhu Sheikh Mujibur Rahman Science & Technology University, Gopalganj 8100, Bangladesh
5Department of Public Health and Institute of Public Health, Chung Shan Medical University, Taichung City 40201, Taiwan
6School of Science, Monash University Malaysia, Jalan Lagoon Selatan, 47500, Bandar Sunway, Selangor Darul Ehsan, Malaysia
- *Corresponding Author:
- Mubarak A khan
Institute of Radiation and Polymer Technology
Bangladesh Atomic Energy Commission
P.O. Box 3787, Dhaka-1000, Bangladesh
E-mail: makhan.inst@gmail.com
Accepted on February 20, 2018
Citation: Jesmin S, Jubayer AAl, Debnath T, et al. Edible transparent coating of irradiated oligo chitosan to preserve aesthetic view and taste of litchi fruit. J Food Microbiol. 2018;2(1):16-21.
Abstract
In this research, the effect of radiation-processed oligo-chitosan on post-harvest loss of litchi fruit was investigated. Different concentrations of irradiated (40 kGy) chitosan solution (500, 1000 and 1500 ppm) were sprayed on litchi fruits both in stalked and stalkless form. Then the treated and untreated litchis were stored in different atmospheric conditions such as at (a) room environment (open and polythene covered) and (b) at 4°C in zip-bag. Fruits coated with irradiated chitosan showed significant delays in the change of weight loss, aesthetic view and microbial count as compared with the uncoated control fruits. The best result was achieved for storage in room temperature at 1500 ppm irradiated chitosan sprayed litchis, where up to 7 days litchis were in good quality. Further improvement was achieved by keeping the litchis in zip lock bag at 4°C. In this case, litchis maintained edible quality with proper aesthetic view up to 21 days. Peeled litchis were also stored at 4°C in air-tight jar in formulated chitosan solution where they maintained edible quality up to 4 months.
Keywords
Litchi fruit, Irradiated chitosan, Radiation, Post-harvest loss, Shelf life, Natural preservative.
Introduction
Litchi (Litchi chinensis Sonn.) is a widely accepted subtropical fruit with significant commercial value. Bangladesh has a good export potential being the second largest producer of litchi in South Asia [1]. Only about 10% of produced litchi can be processed because of its highly perishable nature [2]. The major loss of litchi fruits occurs due to damage during postharvest handling. Both microbial growth and browning enzyme such as peroxidase (POD) and polyphenoloxidase (PPO) can act together to accelerate damage of fruits by taking the advantage of minor injury in pericarp. As a result, quality and aesthetic value is reduced [3,4]. Poor storage facility, non-available cold chain and transportation injury contribute to the postharvest loss of litchi up to 50% [1]. Different studies have been reported on the preservation strategies of whole fruits such as chitosan coating [4]; chilled storage [5]; plastic films [3]; irradiation [1]; controlled atmosphere [6]; anti browning agents, osmo-vacuum drying and moderate vacuum packaging [7]; acid dipping, chemical coatings [8] and thermal processing [9]. Processing of peeled fruit using chitosan coating and subsequent chilled storage is another useful alternative [10].
Chitosan is the second most available natural polymer produced by deacetylating chitin. The major sources are the crustacean shells [11]. The recent commercial applications of chitosan in different sectors; even the use of chitosan in the past decades have made it an attractive mean [12,13]. In these days crab and shrimp shell wastes are used to produce chitosan with different molecular weights and deacetylation grades and thus with different functional properties [11,14]. Chitosan is insoluble in water and soluble in solutions of weak organic acid. Some chitosan derivatives (acetate, lactate, ascorbate, and malate) are water-soluble [15].
Chitosan has the ability to act against the food-borne yeast, bacteria and filamentous fungi and that’s why it is considered as a potential food preservative [16]. Chitosan has also been found to possess a film-forming ability to be used as edible coatings [17-19]. This coating can decrease transpiration, control the internal atmosphere and thus poses a positive effect on fruit’s shelf-life [20,21]. In recent studies chitosan is characterized mostly as a bacteriostatic agent [22]. Yet the full mechanism is not well understood and may be involved with the activities of other molecules [23]. Antimicrobial activity may differ depending on different species as the charges of surface area of related microbes differs [24].
Peeled litchis have priority to consumers due to ease in consuming and serving. But this form is very perishable for lacking pericarp and higher dehydration rate of the open pulp [25]. But edible coating can be helpful for the recovery andminimizing the weight loss of litchi fruits [21,26].
Accordingly, the present study was aimed to reduce the pericarp browning and arial decay of litchi fruits using chitosan. Emphasis was focused on developing the easy, low cost and effective strategies of improving litchi storability during its postharvest storage. Storage of peeled litchis also studied here to find out the probable extendable shelf life by using chitosan.
Materials and Methods
Fruits collection
Mature litchis (variety Bombai) were obtained from a local garden of Shonargaon of Narayanganj near Dhaka, Bangladesh and transported to the laboratory of Institute of Radiation and Polymer Technology (IRPT), Atomic Energy Research Establishment (AERE), Savar, Dhaka (transportation time was 4 hours). Fruits of same variety were selected according to good appearance and free from infection and mechanical injuries.
Preparation of coating formulation
Irradiated Chitosan and edible coating formulations were prepared in the laboratory from prawn shell using chemical extraction method [27]. Degree of deacetylation of the chitosan was 83%. 20 g of chitosan was dissolved in 2% acetic acid in distilled water to prepare 1 L of 2% chitosan solution and subjected to 40 kGy gamma radiation. The gamma irradiation was carried out by Co-60 gamma radiation source situated in IRPT, AERE, Savar, Dhaka at a dose rate of 11.5 kGy per hour.
Application of coating formulation
Coating Application on Litchis was randomly distributed into various groups. One group is in ambient atmosphere both in open air and covered in polyethylene sheet. Other group was in zip-lock bag at refrigerator at 4°C. In case of ambient atmosphere four assemblies were assigned where each to one of four treatments as described in the next sentence whilst the fifth assembly provided untreated control. Samples (with stalk) kept in ambient atmosphere were denoted by LA-1: Control, LA-2: 1000 ppm chitosan sprayed (open), LA-3: 1000 ppm chitosan sprayed (covered) LA-4 1500 ppm chitosan sprayed (open), LA-5 1500 ppm chitosan sprayed (covered). Each assembly comprises with 20 pieces of litchis. Samples kept in refrigerator at 4°C were divided in two groups like litchis with stalk and stalk-less litchi. Samples were denoted by L-1: control, L-2: 500 ppm, L-3: 1000 ppm, L-4: 1500 ppm (litchis with stalk) and L-5: control, L-6: 500 ppm, L-7: 1000 ppm, L-8: 1500 ppm chitosan treated respectively (stalk less). All the samples were covered by polyethylene sheet as uncovered samples were subjected to very rapid moisture loss in the refrigerator. Fruits were washed using distilled water, air dried at room temperature and then irradiated chitosan solutions were applied (by spraying). After the treatment, fruits were air dried and stored at ambient environment (30 ± 1°C / 75 ± 5% RH) up to 7 days and at refrigerator at 4°C for 21 days. Litchis were analyzed for different parameters at a regular interval of 5 days.
Sensory quality evaluation
Sensory evaluation was done to evaluate taste, flavor and aroma of the preserved litchis. A panel of ten judges with age ranging from 25-35 years was selected on their consistency and reliability of judgment. Panelists were asked to rate the difference between samples by allotting the numbers from 0-9, where 0-2 represent extremely dislike, 3-5 for dislike, 6-8 for good and 9 for excellent aroma, taste and flavor.
Other assessments
Biochemical Analysis, Moisture content etc. were determined according to the official methods of analysis of the Association of Official Analytical Chemists (AOAC, 1990) [28].
Microbiological Analysis
Microbiological analysis was conducted by the means of total bacterial count and total fungal count. Plate count agar media (Oxoid, UK) was used for total bacterial count and potato dextrose agar media (Oxoid, UK) was used for the fungal count. Pour plate technique was used for both of the test (APHA, 1992) [29-31].
For Peel-off Litchis
Oligo chitosan was prepared by irradiating the chitosan solution by gamma radiation at 40 kGy radiation dose. Then the peel-off litchi samples were dipped into oligo chitosan formulations of different concentrations (10, 30, 50, 70, 100 ppm) in an air tight jar and were stored at 4°C. Litchi fruit samples dipped in sterile water were served as control.
Result and Discussion
Weight loss percentage at ambient atmosphere
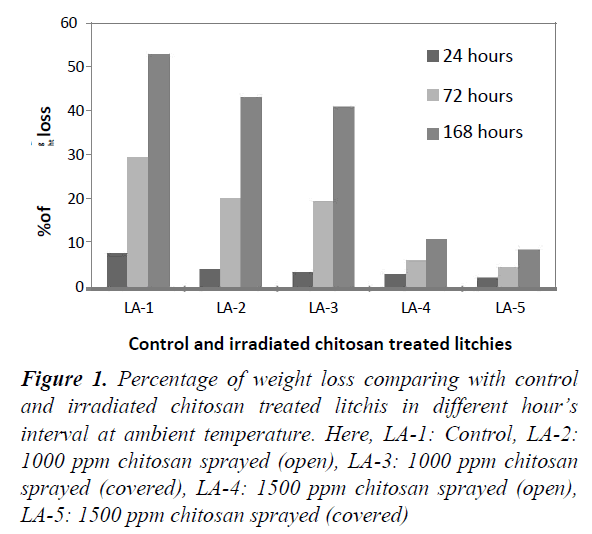
Weight loss was calculated by subtracting the weight of litchi fruits after preservation from the initial weight loss of the fruit. Figure 1 showed the percentage of weight loss for control and irradiated chitosan treated stalked litchis at ambient temperature at a tropical season in Bangladesh. It was noticed that percentage of weight loss increased with the course of time duration. In case of control litchis (LA-1) weight loss reached up to 52.94% after 168 hours, which was the highest comparing with other irradiated chitosan treated litchis. In this period, 1500 ppm irradiated chitosan sprayed covered (LA-5) litchis had only 8.37% loss. The prevention of weight loss was mediated by the two factors i.e. 1. Irradiated chitosan prevented fungal growth on the peel and thus maintained its integrity and 2. Chitosan formed a coating around the fruits which reduced the water vapor loss from the fruits.
Figure 1. Percentage of weight loss comparing with control and irradiated chitosan treated litchis in different hour’s interval at ambient temperature. Here, LA-1: Control, LA-2: 1000 ppm chitosan sprayed (open), LA-3: 1000 ppm chitosan sprayed (covered), LA-4: 1500 ppm chitosan sprayed (open), LA-5: 1500 ppm chitosan sprayed (covered)
Weight loss percentage at 4°C in Zip-lock bag (stalked and stalk-less)
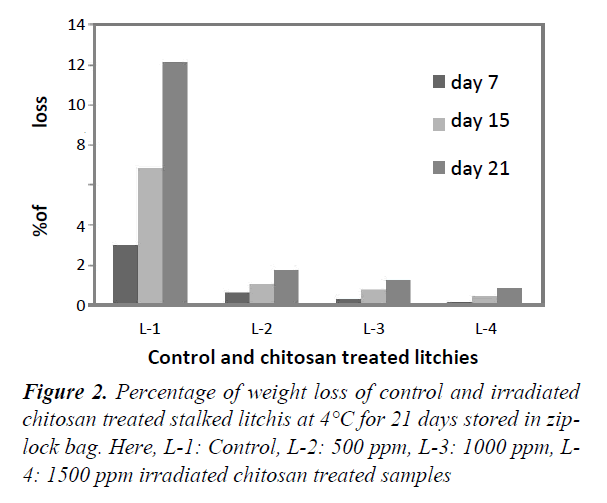
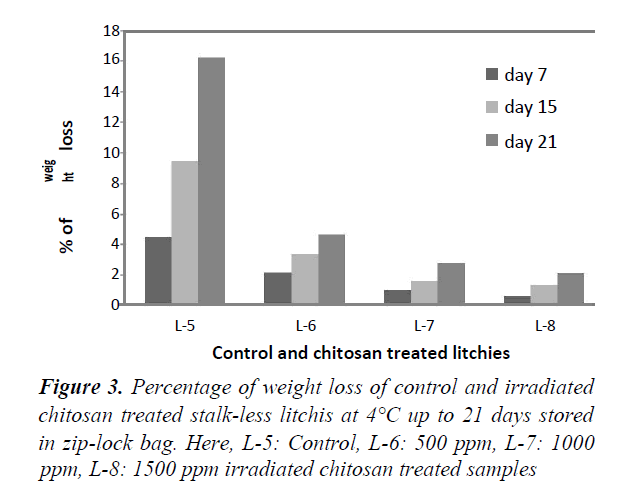
Figures 2 and 3 represent the percentage of weight loss for both stalked and stalk-less litchis stored in zip-lock bags for 21 days at 4°C. For both of the stalked (Figure 1), and stalk-less litchis (Figure 2) it was observed that weight loss increased gradually with the increase of time. But in case of stalked litchis weight loss was lower than stalk-less litchis. After 21 days weight loss was 12% and 16% for control stalked (L-1) and stalk-less (L-5) litchis, respectively. In that period the lowest weight lossshowed for 1500 ppm irradiated chitosan treated litchis (L-4 & L-8) and that were about 1% and 2% only. This was becauseirradiated chitosan made a coating around the fruits. Comparingwith stalked and stalk-less litchis it is clear that stalk less litchisloss more weight because of higher evaporation of moisture,polyphenols and flavoring materials through the peel rupture thatoccurred during removing the stalk (Figure 4).
This protection against the weight loss supports the study of Tamer and L Copur who worked with 1% raw chitosan solution in various fruits [32]. Kumari also found similar result while treating litchis with 2% chitosan at 4°C. But Sun found only 2% less weight loss in litchi fruits [4,33]. The study of Ibrahim supports this moisture protection capability that treated pineapples with irradiated chitosan. Lin reported that respiration rate of litchis were reduced by using edible coating generated by 1% chitosan [34,35].
Decay loss
Table 1 depicts decay and microbial attack on control and chitosan treated litchis at ambient temperature after 48, 72 and 168 hours of treatment. It was found that after 168 hours, all control (LA-1) and 500 ppm chitosan treated litchis (LA-2) were dark in color and infected by microbes. On the other hand, only 3 out of 10 litchis were infected by microbes in case of 1500 ppm chitosan treated (LA-5) litchis within the same period of time. It is suggested that coating with irradiated chitosan acted as an antimicrobial agent and thus lowered the infection. Chitosan coating also helped to preserve the fruit's natural color by preventing moisture loss from the peel as well as from oxidation and thus increased shelf life.
| Litchis | 48 hours | 72 hours | 168 hours |
|---|---|---|---|
| Number of litchis turned dark or attacked by microbes | |||
| *LA-1 | 7 | 9 | all |
| LA-2 | 4 | 6 | all |
| LA-3 | 1 | 3 | 7 |
| LA-4 | 1 | 2 | 5 |
| LA-5 | 1 | 2 | 3 |
*LA = Litchi sample
Table 1. Decay loss of control and irradiated chitosan treated litchis at ambient temperature
Experimental results regarding aesthetic view and microbial attack on control and irradiated chitosan treated stalked and stalk-less litchis at 4°C temperature stored in zip-lock bag is presented in Table 2. Data was taken after 7, 15 and 21 days of interval. It was found that all control (L-1&L-5) and 500 ppm irradiated chitosan treated litchis (L-2&L-6) were infected by microbes after 21 days. But in this period of time only 5 out of 10 litchis and 6 out of 10 litchis were infected by microbes in case of 1500 ppm chitosan treated stalked (L-4) and stalk-less (L-8) litchis respectively. Results suggested that stalk-less litchis were more susceptible to microbial attack than stalked litchis. This was mainly due to the opening on the peel at stalk-less condition. Besides, stalk-less litchis lost moisture more rapidly than the stalked litchis that led to cracking of the peel which might induce microbial infection.
| Litchis | Day 7 | Day 15 | Day 21 | Litchis | Day 7 | Day 15 | Day 21 |
|---|---|---|---|---|---|---|---|
| Number of litchis (stalked) turned dark or attacked by microbes | Number of litchis (stalk-less) turned dark or attacked by microbes | ||||||
| *L-1 | 6 | 8 | all | L-5 | 7 | 9 | all |
| L-2 | 4 | 6 | all | L-6 | 5 | 7 | all |
| L-3 | 1 | 3 | 6 | L-7 | 2 | 4 | 7 |
| L-4 | 1 | 2 | 5 | L-8 | 3 | 5 | 6 |
*L= Litchi Sample
Table 2. Decay loss of control and irradiated chitosan treated litchis at 4°C temperature in zip-lock bag
Microbial count reduction
Experimental data represented that the bacterial count of untreated litchis was 3.68 × 105 cfu/g whereas the bacterial count was 5 × 104 cfu/g in case of chitosan treated litchis. On the other hand, the fungal count of untreated litchis was 8 × 102 cfu/g whereas the fungal count was 40 cfu/g in case of chitosan treated litchis (Table 3). The value shows that irradiated chitosan coating significantly reduced the microbial count in stored litchis. The antimicrobial film forming capacity found in the current study coincided with Tamer and LCopur’s result. Chien and Chou experimented on some citrus fruits and Chien studied on mango. They also found this natural polymer as a potent antimicrobial agent like the present study on litchis [32,36,37].
| Litchis | Colony count on plate count agar media (for bacteria) at different dilutions | Total count | |||
|---|---|---|---|---|---|
| 1-Oct | 2-Oct | 3-Oct | 4-Oct | ||
| Control | TNTC | TNTC | 46 × 8 | 40 × 2 | 3.68 × 105 cfu/g |
| Chitosan treated (LA-5) | TNTC | TNTC | 5 | 1 | 5 × 104 cfu/g |
| Colony count on potato dextrose agar media (for fungus) at different dilutions | |||||
| Control | 37 × 2 | 8 | ND | ND | 8 × 102 cfu/g |
| Chitosan treated (LA-5) | 4 | 1 | ND | ND | 40 cfu/g |
Here 'cfu' represents 'colony forming unit', 'TNTC' represents 'too numerous to count' and 'ND' represents 'not detected'.
Table 3. Bacterial and fungal count of control and chitosan treated litchis after 3 days of storage in room temperature
Preservation of Peel-off Litchis
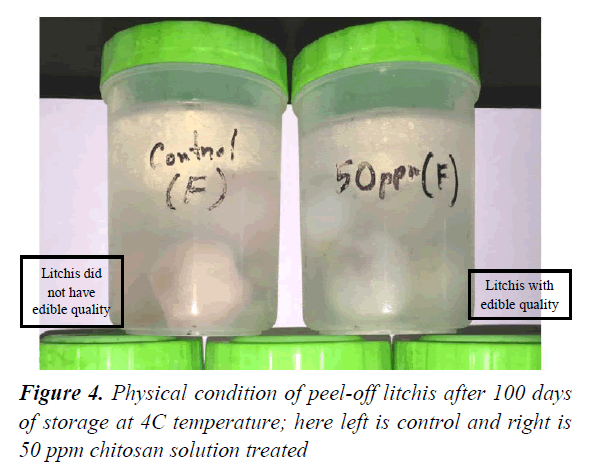
Peel-off litchis stored in chitosan solution showed very inspiring results. Decay rate was found significantly lower than that of control sample. Litchis in 50 ppm irradiated chitosan solution showed best result. The preserved litchis maintained edible condition as well as excellent integrity and aroma up to 4 months whereas control samples spoiled within 15 days. Experimental results showed that irradiated chitosan reduced microbial counts significantly in both of the preservation medium and peeled litchis. Data suggested that irradiated chitosan solution performed better activity against fungus better than the bacteria, Corbo also reported chitosan’s feasibility as an antimicrobial agent for food products which supports the findings of our present study (Table 4) [38].
| Sample | Concentration of chitosan solution | Total bacterial count (after 4 months of Preservation) | Total fungal count (after 4 months of Preservation) |
|---|---|---|---|
| Flesh of preserved fruit | 0 ppm/Control | 3.68 × 105 cfu/g | 8.3 × 103 cfu/g |
| 10 ppm | 5.5 × 104 cfu/g | <10 cfu/g | |
| 30 ppm | 5.3 × 104 cfu/g | <10 cfu/g | |
| 50 ppm | 9 × 103 cfu/g | <10 cfu/g | |
| 70 ppm | 4.5 × 104 cfu/g | <10 cfu/g | |
| 100 ppm | 6 × 104 cfu/g | <10 cfu/g | |
| Liquid media of preserved fruits | 0 ppm/Control | 2.56 × 106 cfu/g | 6.2 × 105 cfu/g |
| 10 ppm | 3.1 × 102 cfu/g | <10 cfu/g | |
| 30 ppm | 1.4 × 102 cfu/g | <10 cfu/g | |
| 50 ppm | 90 cfu/g | <10 cfu/g | |
| 70 ppm | 87 cfu/g | <10 cfu/g | |
| 100 ppm | 5.8 × 102 cfu/g | <10 cfu/g |
Table 4. Microbial count of control and chitosan treated peel-off litchis preserved in jar at 4°C
The microbial inhibition by chitosan may be explained by two interferences. One can be the inner osmotic imbalances by changing the permeability of microbe’s cell wall and thus hindering the microbial growth [39]. Another cause can be the interaction of chitosan with peptidoglycans of the microbial cell wall and leading to the dispersion of internal electrolytes and other necessary molecules [40].
So irradiated chitosan played active roles in inhibiting dehydration and microbial penetration and thus maintaining integrity which led to improved litchi storability.
Conclusion
The overall study indicates that 1500 ppm chitosan irradiated by 40 kGy radiation is very suitable preservation of litchi fruits both in ambient atmosphere and 4°C temperature. More longevity for consumption can be achieved by covering the litchis and lowering the temperature. we was also found that peel-off litchis can be a great source of off-season litchi source if it can be preserved by 50 ppm 40 kGy irradiated chitosan in air-tight jar at 4°C. Thus it can be concluded that radiation processed chitosan is a very suitable for shelf-life extension of litchis and may find a potent application to reduce post-harvest loss of litchis as well as in the food processing industries.
References
- Hajare SN, Saxena S, Kumar S, et al. Quality profile of litchi (Litchi chinensis) cultivars from India and effect of radiation processing. Radiat Phys Chem. 2010;79(9):994-1004.
- Kaushik N, Kaur BP, Rao PS. Application of high pressure processing for shelf life extension of litchi fruits (Litchi chinensis cv. Bombai) during refrigerated storage. Food Sci Technol Int. 2014;20(7):527-41.
- Somboonkaew N, Terry LA. Influence of temperature and packaging on physiological and chemical profiles of imported litchi fruit. Food Res Int. 2011;44(7):1962-69.
- Sun D, Liang G, Xie J, et al. Improved preservation effects of litchi fruit by combining chitosan coating with ascorbic acid treatment during postharvest storage. Afr J Biotechnol. 2010;9(22):3272-79.
- Khan AS, Ahmad N, Malik AU, et al. Cold Storage Influences the Postharvest Pericarp Browning and Quality of Litchi. Int J Agric Res. 2012;14(3):389-94.
- Sivakumar D, Terry LA, Korsten L. An overview on litchi fruit quality and alternative postharvest treatments to replace sulfur dioxide fumigation. Food Rev Int. 2010;26(2):162-188.
- Shah NS, Nath N. Changes in qualities of minimally processed litchis: Effect of antibrowning agents, osmo-vacuum drying and moderate vacuum packaging. Lwt Food Sci Technol. 2008; 41(4):660-68.
- Shah NS, Nath N. Minimally processed fruits and vegetables-freshness with convenience. J Food Sci Tech Mys. 2006; 43(6): 561-70.
- Yu K, Wu Y, Hu Z, et al. Modeling thermal degradation of litchi texture: Comparison of WeLL model and conventional methods. Food Res Int. 2011;44(7):1970-76.
- Dong AJ, Feng MH, Qi HY, et al. Synthesis and properties of O-carboxymethyl chitosan/methoxy poly (ethylene glycol) graft copolymers. J Mater Sci Mater Med. 2008;19(2):869-76.
- No HK, Meyers SP. Preparation and characterization of chitin and chitosan- a review. J Aquat Food Prod T. 1995; 4(2):27-52.
- Dar TA, Khan MA, Uddin M, et al. Effect of Co-60 gamma irradiated chitosan and phosphorus fertilizer on growth, yield and trigonelline content of Trigonella foenum-graecum L. J Radiat Res Appl Sci. 2015;8(3):446-58.
- Knorr D. Use of chitinous polymers in food: a challenge for food research and development. Food Tech. (USA), 1984.
- Cho YI, No HK, Meyers SP. Physicochemical characteristics and functional properties of various commercial chitin and chitosan products. J Agric Food Chem. 1998;46(9):3839-43.
- Jeon YJ, Park PJ, Kim SK. Antimicrobial effect of chit oligosaccharides produced by bioreactor. Carbohydr Polym. 2001;44(1):71-76.
- Sagoo S, Board R, Roller S. Chitosan inhibits growth of spoilage micro-organisms in chilled pork products. Food Microbiol. 2002;19(2-3):175-82.
- Butler BL, Vergano PJ, Testin RF, et al. Mechanical and barrier properties of edible chitosan films as affected by composition and storage. J Food Sci. 1996;61(5):953-56.
- Jeon YJ, Kamil JY, Shahidi F. Chitosan as an edible invisible film for quality preservation of herring and Atlantic cod. J Agric Food Chem. 2002;50(18):5167-78.
- Nadarajah K, Prinyawiwatkul W, Hong K, et al. Sorption behavior of crawfish chitosan films as affected by chitosan extraction processes and solvent types. J Food Sci. 2006;71(2).
- Ghaouth AE, Arul J, Ponnampalam R, et al. Chitosan coating effect on storability and quality of fresh strawberries. J Food Sci. 1991;56(6):1618-20.
- Zhang D, Quantick PC. Effects of chitosan coating on enzymatic browning and decay during postharvest storage of litchi (Litchi chinensis Sonn.) fruit. Postharvest Biol Technol. 1997;12(2):195-202.
- Coma M, Rtial-Gros V, Grreau A, et al. Edible antimicrobial films based on chitosan matrix. J Food Sci. 2002;67(3):1162-69.
- Raafat D, Bargen K, Haas A, et al. Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol. 2008;74(12):3764-73.
- Masson M, Holappa J, Jarvinen T, et al. Antimicrobial activity of piperazine derivatives of chitosan. Carbohydr Polym. 2008; 74(3):566-71.
- Jiang YM, Fu JR. A review of advances in the study of postharvest physiology and technology of storage and transport of litchi fruit. J Subtropic Plant Sci. 2000;3:14.
- Li P, Barth MM. Impact of edible coatings on nutritional and physiological changes in lightly-processed carrots. Postharvest Biol Technol. 1998;14(1):51-60.
- Rashid TU, Rahman MM, Kabir S, et al. A new approach for the preparation of chitosan from γ‐irradiation of prawn shell: effects of radiation on the characteristics of chitosan. Polym Int 2012;61(8):1302-08.
- Chemists AA. Official methods of analysis of AOAC Int. 1990; 1: 15th ed, Arlington, VA.
- Lightfoot NF, Maier EA. Microbiological analysis of food and water: Guidelines for quality assurance. 1998: Elsevier.
- Akther S, Debnath T, Chowdhury MMH. Multidrug Resistant E. coli in Hospital Waste Water: A Potential Concern for Public Health. Adv Biotech Microbiol. 2018;8(1).
- Debnath T, Bhowmik S, Bhowmik S, et al. Presence of multidrug resistant bacteria on mobile phones of healthcare workers accelerates the spread of nosocomial infections and regarded as a threat to public health in Bangladesh. J Microsc Ultrastruct. 2017.
- Tamer CE, Çopur OU. Chitosan: an edible coating for fresh-cut fruits and vegetables. VI International Postharvest Symposium. 2009; 877.
- Kumari P, Barman K, Patel VB, et al. Reducing postharvest pericarp browning and preserving health promoting compounds of litchi fruit by combination treatment of salicylic acid and chitosan. Scientia Horticulturae. 2015;197:555-63.
- Ibrahim SM, Nahar S, Khan MA, et al. Effect of low molecular weight chitosan coating on physico-chemical properties and shelf life extension of pineapple (Ananas sativus). J For Prod Indus. 2014; 3(3): 161-66.
- Lin B, Du Y, Liang X, et al. Effect of chitosan coating on respiratory behavior and quality of stored litchi under ambient temperature. J Food Eng. 2011;102(1):94-99.
- Chien PJ, Chou CC. Antifungal activity of chitosan and its application to control post‐harvest quality and fungal rotting of Tankan citrus fruit (Citrus tankan Hayata). J Sci Food Agric. 2006;86(12):1964-69.
- Chien PJ, Sheu F, Yang FH. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J Food Eng. 2007;78(1):225-29.
- Corbo MR, Bevilacqua A, Campaniello D, et al. Prolonging microbial shelf life of foods through the use of natural compounds and non‐thermal approaches- a review. Int J Food Sci Tech. 2009;44(2):223-41.
- Shahidi F, Arachchi JKV, Jeon YJ. Food applications of chitin and chitosans. Trends Food Sci Technol. 1999;10(2):37-51.
- Devlieghere F, Vermeulen A, Debevere J. Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbial. 2004;21(6):703-14.